Ancool- SF Suspension
Therapy Area
Gastrointestinal
1.0 Generic name
Sucralfate Suspension
2.0 Qualitative and quantitative composition
Composition:
Each 5 ml contains:
Sucralfate IP……………… 500 mg
Colour: Carmoisine
3.0 Dosage form and strength
Suspension, 500mg/5ml
4.0 Clinical particulars
4.1 Therapeutic Indication
Indicated in the treatment of gastric, duodenal and benign ulcer.
4.2 Posology and Method of Administration
The recommended adult oral dosage for duodenal ulcer is 1 gram (10ml) four times per day.
Maximum daily dose: 8 grams.
Ancool®-SF suspension should be administered on an empty stomach on an empty stomach.
Four to six weeks’ treatment is usually needed for ulcer healing, but up to twelve weeks may be necessary in resistant cases.
Antacids may be used as required for relief of pain, but should not be taken half an hour before or after Ancool®-SF suspension.
Paediatric population: The safety and efficacy of Ancool®-SF suspension in children under 14 years of age has not been established.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients
4.4 Special warnings and precautions for use
- Ancool®-SF must not be administered intravenously. Inadvertent intravenous administration of insoluble sucralfate and its insoluble excipients may induce fatal complications, including pulmonary and cerebral emboli. Other severe complications including aluminium intoxication are reported after intravenous administration.
- The product should only be used with caution in patients with renal dysfunction, due to the possibility of increased aluminium absorption.
- Sucralfate is not recommended for use in individuals on dialysis.
- In patients with severe or chronic renal impairment, Ancool®-SF should be used with extreme caution and only for short-term treatment. Small amounts of aluminium are absorbed through the gastrointestinal tract and aluminium may accumulate. Aluminium osteodystrophy, osteomalacia, encephalopathy, and anaemia have been reported in patients with chronic renal impairment. For patients with impairment of renal function, laboratory testing such as aluminium, phosphate, calcium, and alkaline phosphatase is recommended to be periodically performed due to excretion impairment.
- The concomitant use of other aluminium containing medications is not recommended in view of the enhanced potential for aluminium absorption and toxicity. Bezoars have been reported after administration of sucralfate mainly to severely ill patients in intensive care units. The majority of these patients (including neonates in whom sucralfate is not recommended) had underlying conditions that may predispose to bezoar formation (such as delayed gastric emptying due to surgery, drug therapy or diseases that reduce motility), or were receiving concomitant enteral tube feeding.
4.5 Drugs interactions
- Concomitant administration of Ancool®-SF may reduce the bioavailability of certain drugs including fluoroquinolones such as ciprofloxacin and norfloxacin, tetracycline, ketoconazole, sulpiride, digoxin, warfarin, phenytoin, theophylline, levothyroxine, quinidine and H2 antagonists. The bioavailability of these agents may be restored by separating the administration of these agents from Ancool®-SF by two hours. This interaction appears to be non-systemic in origin presumably resulting from these agents being bound by Ancool®-SF in the gastrointestinal tract. Because of the potential of Ancool®-SF to alter the absorption of some drugs from the gastrointestinal tract, the separate administration of Ancool®-SF from that of other agents should be considered when alterations in bioavailability are felt to be critical for concomitantly administered drugs.
- Sucralfate should not be co-administered with citrate preparations. Co-administration of citrate preparations with sucralfate may increase the blood concentrations of aluminium. The mechanism may be due to chelation of aluminium, which is assumed to increase its absorption.
- The administration of Ancool®-SF and enteral feeds by nasogastric tube should be separated by one hour in patients receiving Ancool®-SF Suspension for the prophylaxis of stress ulceration. In rare cases bezoar formation has been reported when Ancool®-SF and enteral feeds have been given too closely together.
4.6 Use in special populations
Pregnancy
Teratogenicity studies in mice, rats and rabits at dose up to 50 times the human dose have revealed no evidence of harm to the foetus. Safety in pregnant women has not been established and Ancool®-SF should be used during pregnancy only if clearly needed.
Lactation:
It is not known whether this drug is excreted in human milk. Caution should be exercised when Ancool®-SF Suspension is administered to breast-feeding women.
4.7 Effects on ability to drive and use machines
There are no data on the effect of this product on driving capacity and use of machines. Do not drive if you feel dizzy or drowsy.
4.8 Undesirable effects
Tabulated list of adverse reactions
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website link: https://www.zuventus.com/drug-safety-reporting
4.9 Overdose
In a clinical trial on healthy men of overdose with sucralfate, most cases remained asymptomatic, but symptoms of abdominal pain, nausea, and vomiting were reported in a few cases.
Acute oral toxicity studies in animals, using doses up to 12 g/kg body weight, could not find a lethal dose. Risks associated with overdose, should, therefore, be minimal.
5.0 Pharmacological properties
Pharmacotherapeutic group: Alimentary tract and metabolism, ATC code: A02B X02
5.1 Pharmacodynamic properties/ Mechanism of Action
Mechanism of action
The action of Ancool®-SF Suspension is non-systemic as the drug is only minimally absorbed from the gastro-intestinal tract. The small amounts that are absorbed are excreted primarily in the urine. Sucralfate exerts a generalised cytoprotective effect by preventing gastro-intestinal mucosal injury.
Studies in humans and animal models show that Sucralfate forms an ulcer adherent complex with the proteinaceous exudate of the ulcer site. This property enables sucralfate to form a protective barrier over the ulcer lesion giving sustained protection against the penetration and action of gastric acid, pepsin and bile. Studies both in humans and animals demonstrate that Sucralfate protects the gastric mucosa against various irritants such as alcohol, acetylsalicylic acid and sodium taurocholate.
Sucralfate also directly inhibits pepsin activity and absorbs bile salts. It has only weak antacid activity. It does not alter gastric emptying time, nor normal digestive function. Sucralfate has no demonstrated pharmacological effect on the cardiovascular or central nervous systems.
5.2 Pharmacokinetic properties
Sucralfate is only minimally absorbed from the gastro-intestinal tract. The small amounts that are absorbed are excreted primarily in the urine. Absorption of aluminium from sucralfate may be increased in patients on dialysis or with renal dysfunction.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
There was no evidence of carcinogenesis in mice and rats receiving oral sucralfate in dosages of up to 1g/kg daily (12 times the usual human dosage) for 2 years. In animal studies there was no effect evidence of impaired fertility. The effect of sucralfate on human fertility is not known.
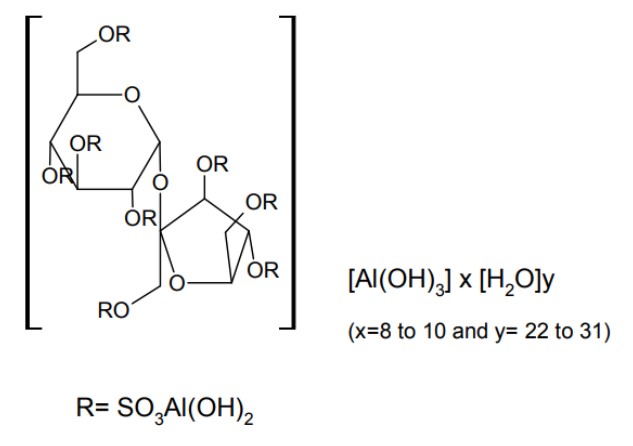
7.0 Description
Ancool®-SF Suspension contain sucralfate and sucralfate is an α-D-glucopyranoside, β-Dfructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex.
Ancool®-SF Suspension for oral administration contains 0.5 g of sucralfate per 5 mL. Therapeutic category: antiulcer.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on the pack
8.3 Packaging information
200 ml bottle, Sugar free
8.4 Storage and handing instructions
Store protected from light and moisture at a temperature not exceeding 30°C.
Keep out of reach of children.
SHAKE WELL BEFORE USE
9.0 Patient counselling information
- Take Ancool®-SF Suspension on an empty stomach, preferably 1 hour before a meal.
- Do not take antacids 30 minutes before or after taking this medication.
- Use caution while driving or doing anything that requires concentration as Sucralfate can cause dizziness and sleepiness.
- It may take 4-6 weeks or more for the ulcers to heal completely. Do not stop taking the medicine until your doctor tells you to.
- Episodes of hyperglycemia have been reported in diabetic patients. Close monitoring of glycemia in diabetic patients treated with sucralfate suspension is recommended. Adjustment of the antidiabetic treatment dose during the use of sucralfate suspension might be necessary.
12.0 Date of revision
July 2024
About leaflet
This product is available as the above name but will be referred to as Ancool®-SF Suspension throughout this leaflet.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effect not listed in this leaflet. See section 4.
What is in this leaflet
- What Ancool®-SF Suspension is and what' it is used for
- What you need to know before you take Ancool®-SF Suspension
- How to take Ancool®-SF Suspension
- Possible side effects
- How to store Ancool®-SF Suspension
- Contents of the pack and other information
1. What Ancool®-SF Suspension is and what it is used for
Ancool®-SF Suspension contains a medicine called sucralfate. This belongs to a group of medicines called "Ulcer-healing" medicines. It works by forming a protective barrier over a stomach ulcer and helps stop further irritation caused by stomach acid.
Ancool®-SF Suspension is used to treat:
- Stomach ulcers
- Ulcers in your bowel
- Inflammation of your stomach lining
Ancool®-SF Suspension can also be used to prevent bleeding ulcers in seriously ill patients in hospital.
2. What you need to know before you take Ancool®-SF Suspension
Do not take Ancool®-SF Suspension:
- If you are allergic (hypersensitive) to sucralfate or any of the other ingredients of Ancool®-SF
- Suspension. Signs of an allergic reaction include: a rash, swallowing or breathing problems,
- swelling of your lips, face, throat or tongue
- If you are on dialysis for kidney problems
- Do not take this medicine if any of the above apply to you. If you are not sure talk to your doctor or pharmacist before taking Ancool®-SF Suspension.
- You must not be given this medicine by injection into a vein.
Warnings and precautions
Talk to your doctor or pharmacist before taking Ancool®-SF Suspension if:
- You are pregnant, trying to become pregnant or breast-feeding (see 'Pregnancy/breast-feeding' below)
- You have kidney problems
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before taking Ancool®-SF Suspension.
Other medicines and Ancool®-SF Suspension
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
This includes medicines obtained without a prescription, including herbal medicines. This is because Ancool®-SF Suspension can affect the way some other medicines work. Also some other medicines can affect the way Ancool®-SF Suspension works.
- Medicines containing citrates
- Medicines containing aluminium
- Medicines for stomach ulcers such as cimetidine or ranitidine
- Medicines for infection (antibiotics) such as tetracycline, ciprofloxacin or norfloxacin
- Sulpiride-used for mental health problems (psychotic episodes)
- Digoxin-used for heart problems
- Phenytoin-used for fits (epilepsy)
- Warfarin-used for blood clots
- Theophylline-used for asthma or other breathing problems
- Levothyroxine-used for thyroid problems
- Quinidine-used for heart problems
- Ketaconazole-used for fungal infections
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before taking Ancool®-SF Suspension.
If you are taking any other medicine, leave a two-hour gap between taking Ancool®-SF Suspension and the other medicine. For "antacid" medicines, the gap needs to be at least half an hour (30 min).
Ancool®-SF Suspension with food and drink
If you are being fed by a tube into your stomach, Ancool®-SF Suspension should be given at least one hour before or after a feed.
Pregnancy and breast-feeding
Talk to your doctor or pharmacist before taking this medicine if:
- You are pregnant, might become pregnant or think you might be pregnant
- You are breast-feeding or planning to breast-feed
Ask your doctor or pharmacist for advice before taking any medicine if you might be pregnant.
Driving and using machines
Ancool®-SF Suspension may make you feel dizzy or drowsy. If you feel dizzy or drowsy, do not drive or use tools or machines while taking this medicine.
3. How to take Ancool®-SF Suspension?
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is: 10 ml spoonful four times a day. Doses should be taken before meals and at bedtime.
In seriously ill patients in hospital, the dose may be increased to 10 ml six times a day.
Taking this medicine
- Take this medicine by mouth
- This medicine is usually taken for 4 to 6 weeks, but may be taken for up to 12 weeks if necessary
- The maximum dose in a day is 8 grams.
Use in children
Sucralfate Suspension is not suitable for children under 14 years old.
If you take more Sucralfate Suspension than you should
If you take more Sucralfate Suspension than you should, talk to a doctor or go to hospital straight away. Take the medicine pack with you. This is so the doctor knows what you have taken.
If you forget to take Sucralfate Suspension
- If you forget a dose, take it as soon as you remember it. However, if it is nearly time for the next dose, skip the missed dose
• Do not take a double dose to make up for a forgotten dose
If you stop taking Sucralfate Suspension
Do not stop taking Sucralfate Suspension without talking to your doctor.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects?
Like all medicines, Sucralfate Suspension can cause side effects, although not everybody gets them.
Stop taking Sucralfate Suspension and tell your doctor straight away if:
- You have an allergic reaction. The signs include a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue
Tell a doctor or pharmacist if any of the following side effects gets serious or lasts longer than a few days:
- Constipation or diarrhoea
- Indigestion, stomach ache or wind
- Being sick (vomiting) or feeling sick (nausea)
- Feeling dizzy, drowsy or being unsteady on your feet
- Back pain
- Dry mouth
- Headache
- Itching
Sucralfate Suspension may cause a blockage in the digestive tract of seriously ill patients in hospital.
This is a rare side effect.
Sucralfate Suspension may cause aluminium to build up in your body. This can cause changes in your bones such as thinning or softening, impairment of your brain or problems with your blood.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: https://www.zuventus.com/ and click the tab “Safety Reporting” located on the top of the home page.
https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ancool®-SF Suspension.
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and on the bottle label. The expiry date refers to the last day of that month.
Do not store above 30°C.
Once opened the medicine should be used within 28 days.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throwaway any medicines you no longer use. These measures will help to protect the environment.
If your medicine becomes discoloured or shows any other signs of deterioration consult your pharmacist who will tell you what to do.
6. Contents of the pack and other information
What Sucralfate Suspension contains
- Sucralfate Suspension contains the active substance sucralfate. Each 5ml dose contains 500 mg sucralfate.
- Colour: Carmoisine
Packaging information
200 ml bottle with 5 ml measuring spoon.
(Sugar free)
Marketing Authorisation Holder
Zuventus Healthcare Limited
Zuventus House Plot No. Y2, CTS No: 358/A2,
near Nahur Railway Station, off Raycon IT Park Road,
Nahur West, Mumbai, Maharashtra 400078
Leaflet revision date (Ref.): 04.07.2024.