Augpen ES Oral Suspension
Therapy Area
Anti Infective
1.0 Generic name
Amoxycillin and Potassium Clavulanate Oral Suspension IP
2.0 Qualitative and quantitative composition
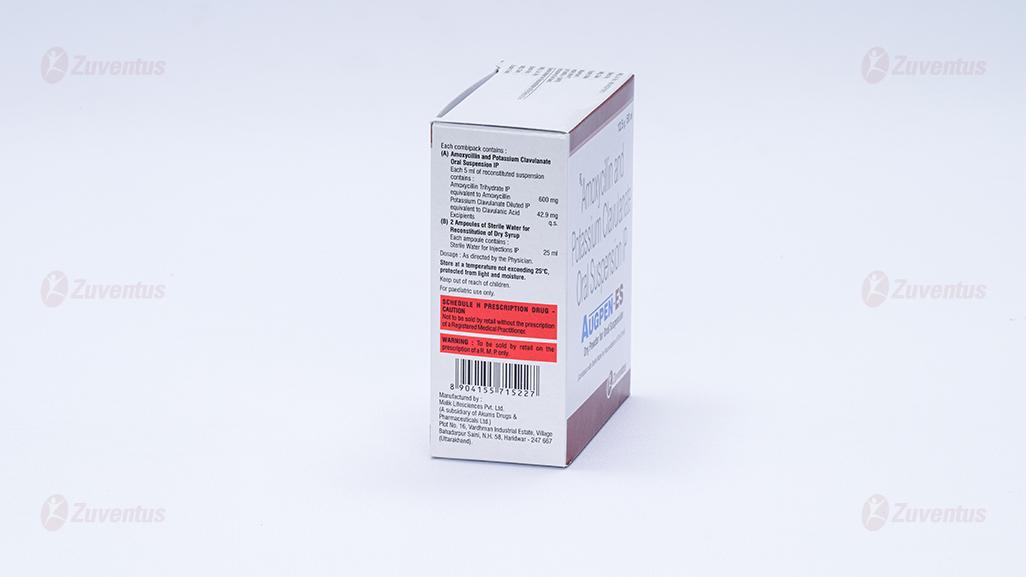
Each 5 ml of reconstituted suspension contains :
Amoxycillin Trihydrate IP
equivalent to Amoxycillin 600 mg
Potassium Clavulanate Diluted IP
equivalent to Clavulanic Acid 42.9 mg
Excipients q.s
3.0 Dosage form and strength
Oral Suspension
4.0 Clinical particulars
4.1 Therapeutic indication
It is indicated for short term treatment of pediatric patients with bacterial infections at the following sites when caused by Amoxycillin-Clavulanate-susceptible organisms :
- Upper Respiratory Tract Infections (including ENT) e.g.
- Acute otitis media (AOM), persistent AOM, or recurrent AOM, typically caused by Streptococcus pneumonia, Haemophilus infuenza and Moraxella catarrhalis.
- Tonsillo-pharyngitis and sinusitis, typically caused by Streptococcus pneumonia, Haemophilus Infuenza, Moraxella catarrhalis and Streptococcus pyogenes
- Lower Respiratory Tract Infections e.g. lobar and bronchopneumonia typically caused by Streptococcus pneumonia, Haemophilus infuenza and Moraxella catarrhalis.
4.2 Posology and method of administration
Dosage should be expressed in terms of the age of the child and either in mg/kg/day or ml of suspension per dose. Dosages are expressed throughout in terms of Amoxycillin / Clavulanate content except when doses are stated in terms of an individual component. To minimize potential gastrointestinal intolerance, administer at the start of a meal. The absorption of Amoxycillin-Clavulanate is optimized when taken at the start of a meal. Therapy can be started parenterally and continued with an oral preparation. Treatment should not be extended beyond 14 days without review.
Adults There is no experience with Augpen ES in adults.
Children
Augpen ES is recommended for dosing at 90/6.4 mg/kg/day in 2 divided doses at 12-hourly intervals for 10 days.
There is no experience in paediatric patients weighing more than 40 kg.
| Body Weight (kg) | Volume of Augpen ES providing 90/6.4 mg/kg/day |
| 8 | 3 ml twice daily |
| 12 | 4.5 ml twice daily |
| 16 | 6 ml twice daily |
| 20 | 7.5 ml twice daily |
| 24 | 9 ml twice daily |
| 28 | 10.5 ml twice daily |
| 32 | 12 ml twice daily |
| 36 | 13.5 ml twice daily. |
| 40 | 15 ml twice daily. |
Renal impairment
No dosage adjustment is necessary in patients with creatinine clearance of greater than or equal to 30 ml/min. There are no dosage recommendations available for Augpen ES in patients with creatinine clearance of less than 30 ml/min.
Hepatic impairment
Dose with caution; monitor hepatic function at regular intervals. There are insuffcient data on which to base a dosage recommendation
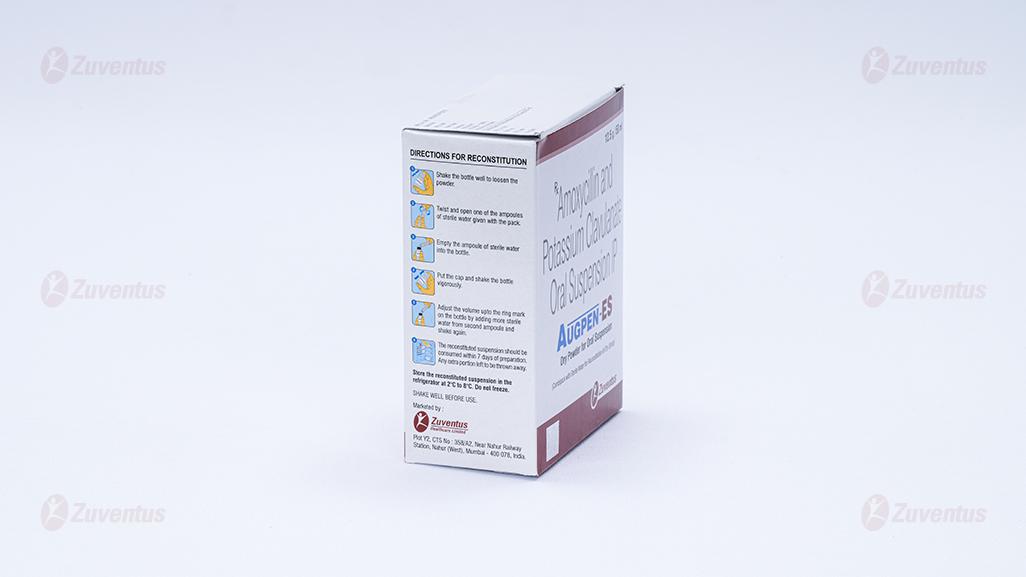
Directions for reconstitution
Shake the bottle well to loosen the powder. Slowly add freshly boiled & cooled water up to the ring mark on the bottle. Put the cap & shake the bottle vigorously. If required adjust the volume upto the ring mark on the bottle with more water and shake again. This makes 50 ml of the suspension. The reconstituted suspension should be consumed within 7 days of preparation. Any extra portion left to be thrown away.
4.3 Contraindications
- In patients with a history of hypersensitivity to beta-lactams, e.g. penicillins and cephalosporins.
- In patients with a previous history of Amoxycillin-Clavulanate associated jaundice/hepatic dysfunction.
4.4 Special warnings and precautions for use
- Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. These reactions are more likely to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins
- Before initiating therapy with Amoxycillin and Potassium Clavulanate for Oral Suspension, 600 mg/42.9 mg per 5 ml careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg / 42.9 mg per 5 ml should be discontinued and the appropriate therapy instituted. Serious anaphylactic reactions require immediate emergency treatment with epinephrine. oxygen, intravenous steroids, and airway management, including intubation, should also be administered as indicated.
- Pseudomembranous colitis has been reported with nearly all antibacterial agents, including Amoxycillin/Clavulanate Potassium, and has ranged in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhoea subsequent to the administration of antibacterial agents.
- Treatment with antibacterial agents alters the normal fora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium diffcile is one primary cause of "antibiotic-associated colitis".
- After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fuids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. diffcile colitis
- Amoxycillin and Clavulanate Potassium for Oral Suspension 600 mg / 42.9 mg per 5 ml should be used with caution in patients with evidence of hepatic dysfunction. Hepatic toxicity associated with the use of Amoxycillin/Clavulanate Potassium is usually reversible. On rare occasions, deaths have been reported (less than 1 death reported per estimated 4 million prescriptions worldwide). These have generally been cases associated with serious underlying diseases or concomitant medications.
4.5 Drugs interactions
Concomitant use of probenecid is not recommended. Probenecid decreases the renal tubular secretion of Amoxycillin. Concomitant use with Amoxycillin-Clavulanate may result in increased and prolonged blood levels of Amoxycillin but not of Clavulanic acid.
Concomitant use of Allopurinol during treatment with Amoxycillin can increase the likelihood of allergic skin reactions. There are no data on the concomitant use of AmoxycillinClavulanate and Allopurinol.
In common with other antibiotics, Amoxycillin-Clavulanate may affect the gut fora, leading to lower oestrogen reabsorption and reduced efficacy of combined oral contraceptives.
In the literature there are rare cases of increased international normalised ratio in patients maintained on Acenocoumarol or Warfarin and prescribed a course of Amoxycillin. If co administration is necessary, the prothrombin time or international normalised ratio should be carefully monitored with the addition or withdrawal of Amoxycillin.
In patients receiving Mycophenolate Mofetil, reduction in pre-dose concentration of the active metabolite Mycophenolic acid of approximately 50% has been reported following commencement of oral Amoxycillin plus Clavulanic acid. The change in pre-dose level may not accurately represent changes in overall MPA exposure.
4.6 Use in special populations
Pregnancy
Reproduction studies in animals (mice and rats at doses up to 10 times the human dose) with orally and parenterally administered Amoxycillin-Clavulanate have shown no teratogenic effects. In a single study in women with pre-term, premature rupture of the foetal membrane (pPROM), it was reported that prophylactic treatment with Amoxycillin-Clavulanate may be associated with an increased risk of necrotising enterocolitis in neonates. As with all medicines, use should be avoided in pregnancy, unless considered essential by the Physician.
Nursing mothers
Amoxycillin-Clavulanate may be administered during the period of lactation. With the exception of the risk of sensitization, associated with the excretion of trace quantities in breast milk, there are no known detrimental effects for the breast-fed infant.
4.7 Effects on ability to drive and use machines
Adverse effects on the ability to drive or operate machinery have not been observed.
4.8 Undesirable effects
Amoxycillin/Clavulanate induced Stevenson Johnson Syndrome (SJS)/ Toxic Epidermal Necrosis (TEN).
Infections and infestations
Common : Mucocutaneous candidiasis.
Blood and lymphatic system disorders
Rare:Reversible leucopenia (including neutropenia) and thrombocytopenia
Very rare : Reversible agranulocytosis and haemolytic anaemia. Prolongation of bleeding time and prothrombin time.
Immune system disorders
Very rare : Angioneuotic oedema, Anaphylaxis, Serum sickness-like syndrome, Hypersensitivity vasculitis.
Nervous system disorders
Uncommon: Dizziness, Headache.
Very rare: Reversible hyperactivity, Aseptic meningitis, Convulsions. Convulsions may occur in patients with impaired renal function or in those receiving high doses.
Gastrointestinal disorders
Common: Diarrhoea, Nausea, Vomiting.
Nausea is more often associated with higher oral dosages. If gastrointestinal reactions are
evident, they may be reduced by taking Amoxycillin-clavulanate at the start of a meal.
Uncommon: Indigestion.
Very rare: Antibiotic-associated colitis (including pseudomembranous colitis and haemorrhagic colitis), Black hairy tongue, superficial tooth discolouration has been reported very rarely in children. Good oral hygiene may help to prevent tooth discolouration as it can usually be removed by brushing.
Hepatobiliary disorders
Uncommon: A moderate rise in AST and/or ALT has been noted in patients treated with betalactam class antibiotics, but the significance of these findings is unknown.
Very rare: Hepatitis and cholestatic jaundice. These events have been noted with other penicillins and cephalosporins.
Hepatic events have been reported predominantly in males and elderly patients and may be associated with prolonged treatment. These events have been very rarely reported in children. Signs and symptoms usually occur during or shortly after treatment but in some cases may not become apparent until several weeks after treatment has ceased. These are usually reversible. Hepatic events may be severe and in extremely rare circumstances, deaths have been reported. These have almost always occurred in patients with serious underlying disease or taking concomitant medications known to have the potential for hepatic effects.
Skin and subcutaneous tissue disorders
Uncommon: Skin rash, Pruritus, Urticaria
Rare: Erythema multiforme.
Very rare : Stevenson-Johnson syndrome, Toxic epidermal necrosis, Bullous exfoliativedermatitis, Acute generalised exanthemous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS).
If any hypersensitivity dermatitis reaction occurs, treatment should be discontinued.
Renal and urinary disorders
Very rare: Interstitial nephritis, Crystalluria.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
4.9 Overdose
Symptoms and signs
Gastrointestinal symptoms and disturbance of the fluid and electrolyte balances may be evident. Amoxycillin crystalluria, in some cases leading to renal failure, has been observed.
Treatment
Gastrointestinal symptoms may be treated symptomatically, with attention to the water/electrolyte balance. Amoxycillin-Clavulanate can be removed from the circulation by haemodialysis. A prospective study of 51 paediatric patients at a poison control centre suggested that overdosages of less than 250 mg/kg of Amoxycillin are not associated with significant clinical symptoms and do not require gastric emptying.
5.0 Pharmacological properties
5.1 Mechanism of action
Amoxycillin is a semisynthetic antibiotic with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microorganisms. Amoxycillin is however susceptible to degradation by beta-lactamase and therefore the spectrum of activity of Amoxycillin alone does not include organisms which produce these enzymes. Clavulanic acid is a beta-lactam, structurally related to the penicillins, which possesses the ability to inactivate a wide range of beta-lactamase enzymes commonly found in micro-organisms resistant to penicillins and cephalosporins. In particular, it has good activity against the clinically important plasmid mediated beta-lactamases frequently responsible for transferred drug resistance. It is generally less effective against chromosomally-mediated type 1 beta-lactamases. The presence of clavulanic acid in Amoxycillin-Clavulanate formulations protects Amoxycillin from degradation by beta-lactamase enzymes and effectively extends the antibacterial spectrum of Amoxycillin to include many bacteria normally resistant to Amoxycillin and other penicillins and cephalosporins. Thus Amoxycillin-Clavulanate possesses the distinctive properties of a broad spectrum antibiotic and a beta-lactamase inhibitor
5.2 Pharmacodynamic properties
Amoxycillin/Clavulanic acid has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections.
Aerobic gram-positive microorganisms
Streptococcus pneumonia (including isolates with penicillin MICs ≤ 2 mcg/ml)
Aerobic gram-negative microorganisms
Haemophilusinfluenzae(including β-lactamase-producing isolates) Moraxella catarrhalis (including β-lactamase-producing isolates) The following in vitro data are available, but their clinical signifcance is unknown. At least 90% of the following microorganisms exhibit in vitro minimum inhibitory concentrations (MICs) less than or equal to the susceptible breakpoint for Amoxycillin/Clavulanic acid. However, the safety and efficacy of Amoxycillin/Clavulanic acid in treating infections due to these microorganisms have not been established in adequate and well-controlled trials.
Aerobic gram-positive microorganisms
Staphylococcus aureus (including β-lactamase-producing isolates)
5.3 Pharmacokinetic properties
The pharmacokinetics of Amoxycillin and clavulanate were determined in a study of 19 paediatric patients, 8 months to 11 years, given Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg/42.9 mg per 5 ml at an Amoxycillin dose of 45 mg/kg every 12 hours with a snack or meal. The mean plasma Amoxycillin and Clavulanate pharmacokinetic parameter values are listed in the following table.
Table 1 : Mean (± SD) Plasma Amoxycillin and Clavulanate Pharmacokinetic Parameter Values
Following Administration of 45 mg/kg of Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg/42.9 mg per 5 ml Every 12 Hours to Paediatric Patients.
| Parameter | Amoxycillin | Clavulanate |
| Cmax (mcg/ml) | 15.7 ± 7.7 | 1.7 ± 0.9 |
| Tmax (hr) | 2 (1 - 4) | 1.1(1 - 4) |
| AUC0-t(mcg•hr/ml) | 59.8 ± 20 | 4 ± 1.9 |
| T½ (hr) | 1.4 ± 0.3 | 1.1 ± 0.3 |
| CL/F (L/hr/kg) | 0.9 ± 0.4 | 1.1 ± 1.1 |
The effect of food on the oral absorption of Amoxycillin and Clavulanate Potassium for Oral Suspension 600 mg/42.9 mg per 5 ml has not been studied. Approximately 50% to 70% of the Amoxycillin and approximately 25% to 40% of the Clavulanic acid are excreted unchanged in urine during the first 6 hours after administration of 10 ml of 250 mg/62.5 mg per 5 ml suspension of Amoxycillin and Clavulanate Potassium. Concurrent administration of probenecid delays Amoxycillin excretion but does not delay renal excretion of clavulanic acid.
Neither component in Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg/42.9 mg per 5 ml is highly protein-bound; clavulanic acid has been found to be approximately 25% bound to human serum and Amoxycillin approximately 18% bound.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
No known animal toxicology data.
7.0 Description
Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg/42.9 mg per 5 ml is an oral antibacterial combination consisting of the semisynthetic antibiotic Amoxycillin and the βlactamase inhibitor, Clavulanate Potassium (the Potassium salt of Clavulanic acid).
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack
8.3 Packaging information
A bottle of 12.5 g / 50 ml.
8.4 Storage and handing instructions
Store at a temperature not exceeding 25°C, protected from light & moisture.
Keep out of reach of children
SHALE WELL BEFORE USE
Store the reconstituted suspension in the refrigerator at 2°C to 8°C.
9.0 Patient counselling information
- Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg /42.9 mg per 5 ml should be taken every 12 hours with a meal or snack to reduce the possibility of gastrointestinal upset. If diarrhoea develops and is severe or lasts more than 2 or 3 days, call your doctor.
- Diarrhoea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible
- Keep suspension refrigerated. Shake well before using. When dosing a child with the suspension (liquid) of Amoxycillin and Potassium Clavulanate for Oral Suspension, 600 mg/42.9 mg per 5 ml use a dosing spoon or medicine dropper. Be sure to rinse the spoon or dropper after each use. Bottles of suspension of Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg /42.9 mg per 5 ml may contain more liquid than required. Follow your doctor's instructions about the amount to use and the days of treatment your child requires. Discard any unused medicine.
- Patients should be counselled that antibacterial drugs including Amoxycillin and Potassium Clavulanate for Oral Suspension, 600 mg/42.9 mg per 5 ml should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg/42.9 mg per 5 ml is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may : (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Amoxycillin and Potassium Clavulanate for Oral Suspension 600 mg/42.9 mg per 5 ml or other antibacterial drugs in the future.
12.0 Date of issue
09 November 2022

About leaflet
Read all of this leaflet carefully before you start giving your child this medicine because it contains important information for them.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine is usually prescribed for a baby or child. Do not pass it on to others. It may harm them, even if their signs of illness are the same as your child’s.
- If your child gets any side effects, talk to their doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
- What Augpen ES suspension is and what it is used for
- What you need to know before you give Augpen ES suspension
- How to give Augpen ES suspension
- Possible side effects
- How to store Augpen ES suspension
- Contents of the pack and other information
1. What Augpen ES suspension is and what it is used for
Augpen ES suspension is an antibiotic and works by killing bacteria that cause infections. It contains two different medicines called amoxicillin and clavulanic acid. Amoxicillin belongs to a group of medicines called “penicillins” that can sometimes be stopped from working (made inactive). The other active component (clavulanic acid) stops this from happening.
It is indicated for short term treatment of pediatric patients with bacterial infections at the following sites when caused by Amoxycillin-Clavulanate-susceptible organisms:
- Upper Respiratory Tract Infections (including ENT) e.g.
Acute otitis media (AOM), persistent AOM,
or recurrent AOM Tonsillo-pharyngitis and sinusitis
- Lower Respiratory Tract Infections e.g. lobar and bronchopneumonia.
2. What you need to know before you give Augpen ES suspension
Do not give your child Augpen ES suspension:
- if they are allergic to amoxicillin, clavulanic acid, penicillin or any of the other ingredients of this medicine.
- if they have ever had a severe allergic reaction to any other antibiotic. This can include a skin rash or swelling of the face or throat.
- if they have ever had liver problems or jaundice (yellowing of the skin) when taking an antibiotic.
➔ Do not give Augpen ES suspension to your child if any of the above apply to your child. If you are not sure, talk to their doctor or pharmacist before giving Augpen ES suspension.
Warnings and precautions
Check with their doctor, pharmacist or nurse before giving your child Augpen ES suspension if they:
- have glandular fever
- are being treated for liver or kidney problems
- are not passing water regularly.
If you are not sure if any of the above apply to your child, talk to their doctor or pharmacist before giving Augpen ES suspension.
In some cases, your doctor may investigate the type of bacteria that is causing your child’s infection.
Depending on the results, your child may be given a different strength of Augpen ES suspension or a different medicine.
Conditions you need to look out for
Augpen ES suspension can make some existing conditions worse, or cause serious side effects. These include allergic reactions, convulsions (fits) and inflammation of the large intestine. You must look out for certain symptoms while your child is taking Augpen ES suspension, to reduce the risk of any problems. See ‘Conditions you need to look out for’ in Section 4.
Blood and urine tests
If your child is having blood tests (such as red blood cell status tests or liver function tests) or urine tests (for glucose), let the doctor or nurse know that they are taking Augpen ES suspension. This is because Augpen ES suspension can affect the results of these types of tests.
Other medicines and Augpen ES suspension
Tell your doctor or pharmacist if your child is taking, has recently taken or might take any other medicines.
If your child is taking allopurinol (used for gout) with Augpen ES suspension, it may be more likely that they will have an allergic skin reaction.
If your child is taking probenecid (used for gout), your doctor may decide to adjust the dose of Augpen ES suspension.
If medicines to help stop blood clots (such as warfarin) are taken with Augpen ES suspension then extra blood tests may be needed.
Augpen ES suspension can affect how methotrexate (a medicine used to treat cancer or rheumatic diseases) works.
Augpen ES suspension can affect how mycophenolate mofetil (a medicine used to prevent the rejection of transplanted organs) works.
Pregnancy, breast-feeding and fertility
If your child who is about to take this medicine is pregnant or breast-feeding, thinks they may be pregnant or are planning to have a baby, ask their doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Augpen ES suspension can have side effects and the symptoms may make you unfit to drive. Do not drive or operate machinery unless you are feeling well.
3. How to give Augpen ES suspension
Dosage should be expressed in terms of the age of the child and either in mg/kg/day or ml of suspension per dose. Dosages are expressed throughout in terms of Amoxycillin / Clavulanate content except when doses are stated in terms of an individual component. To minimize potential gastrointestinal intolerance, administer at the start of a meal. The absorption of Amoxycillin-Clavulanate is optimized when taken at the start of a meal. Therapy can be started parenterally and continued with an oral preparation.
Treatment should not be extended beyond 14 days without review.
Adults: There is no experience with Augpen ES in adults.
Children: Augpen ES is recommended for dosing at 90/6.4 mg/kg/day in 2 divided doses at 12-hourly intervals for 10 days.
There is no experience in paediatric patients weighing more than 40 kg.
Renal impairment
No dosage adjustment is necessary in patients with creatinine clearance of greater than or equal to 30 ml/min. There are no dosage recommendations available for Augpen ES in patients with creatinine clearance of less than 30 ml/min.
Hepatic impairment
Dose with caution; monitor hepatic function at regular intervals. There are insufficient data on which to base a dosage recommendation.
Directions for reconstitution
Shake the bottle well to loosen the powder. Slowly add freshly boiled & cooled water up to the ring mark on the bottle. Put the cap & shake the bottle vigorously. If required adjust the volume upto the ring mark on the bottle with more water and shake again. This makes 50 ml of the suspension. The reconstituted suspension should be consumed within 7 days of preparation. Any extra portion left to be thrown away
How to give Augpen ES suspension
- Always shake the bottle well before each dose
- Give with a meal
- Space the doses evenly during the day, at least 4 hours apart. Do not take 2 doses in 1 hour.
- Do not give your child Augpen ES suspension for more than 2 weeks. If your child still feels unwell they should go back to see the doctor.
If you give more Augpen ES suspension than you should
If you give your child too much Augpen ES suspension, signs might include an upset stomach (feeling sick, being sick or diarrhoea) or convulsions. Talk to their doctor as soon as possible. Take the medicine bottle to show the doctor.
If you forget to give Augpen ES suspension
If you forget to give your child a dose, give it as soon as you remember. You should not give your child the next dose too soon, but wait about 4 hours before giving the next dose. Do not take a double dose to make up for a forgotten dose.
If your child stops taking Augpen ES suspension
Keep giving your child Augpen ES suspension until the treatment is finished, even if they feel better. Your child needs every dose to help fight the infection. If some bacteria survive they can cause the infection to come back.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects below may happen with this medicine.
Conditions you need to look out for
Allergic reactions:
- skin rash
- inflammation of blood vessels (vasculitis) which may be visible as red or purple raised spots on the skin, but can affect other parts of the body
- fever, joint pain, swollen glands in the neck, armpit or groin
- swelling, sometimes of the face or throat (angioedema), causing difficulty in breathing
- collapse.
➔ Contact a doctor immediately if your child gets any of these symptoms. Stop taking Augpen ES suspension.
Inflammation of large intestine
Inflammation of the large intestine, causing watery diarrhoea usually with blood and mucus, stomach pain and/or fever.
➔ Contact your doctor as soon as possible for advice if your child gets these symptoms. Very common side effects
These may affect more than 1 in 10 people
- diarrhoea (in adults).
Common side effects
- These may affect up to 1 in 10 people
- thrush (candida - a yeast infection of the vagina, mouth or skin folds)
- feeling sick (nausea), especially when taking high doses
➔ if affected give Augpen ES suspension with a meal
- vomiting
- diarrhoea (in children).
Uncommon side effects
These may affect up to 1 in 100 people
- skin rash, itching
- raised itchy rash (hives)
- indigestion
- dizziness
- headache.
Uncommon side effects that may show up in blood tests:
- increase in some substances (enzymes) produced by the liver.
Rare side effects
These may affect up to 1 in 1000 people
- skin rash, which may blister, and looks like small targets (central dark spots surrounded by a paler area, with a dark ring around the edge - erythema multiforme)
➔ if you notice any of these symptoms contact a doctor urgently.
Rare side effects that may show up in blood tests:
- low number of cells involved in blood clotting
- low number of white blood cells.
Frequency not known
Frequency cannot be estimated from the available data.
- Allergic reactions (see above)
- Inflammation of the large intestine (see above)
- Inflammation of the protective membrane surrounding the brain (aseptic meningitis)
- Serious skin reactions:
- a widespread rash with blisters and peeling skin, particularly around the mouth, nose, eyes and genitals (Stevens-Johnson syndrome), and a more severe form, causing extensive peeling of the skin (more than 30% of the body surface - toxic epidermal necrolysis)
- widespread red skin rash with small pus-containing blisters (bullous exfoliative dermatitis)
- a red, scaly rash with bumps under the skin and blisters (exanthemous pustulosis)
- flu-like symptoms with a rash, fever, swollen glands, and abnormal blood test results (including increased white blood cells (eosinophilia) and liver enzymes) (Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)).
➔ Contact a doctor immediately if your child gets any of these symptoms.
- inflammation of the liver (hepatitis)
- jaundice, caused by increases in the blood of bilirubin (a substance produced in the liver) which may make your child’s skin and whites of the eyes appear yellow • inflammation of tubes in the kidney
- blood takes longer to clot
- hyperactivity
- convulsions (in people taking high doses of Augpen ES suspension or who have kidney problems)
- black tongue which looks hairy
- stained teeth (in children), usually removed by brushing.
Side effects that may show up in blood or urine tests:
- severe reduction in the number of white blood cells
- low number of red blood cells (haemolytic anaemia)
- crystals in urine.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
5. How to store Augpen ES suspension
Keep this medicine out of the sight and reach of children.
Store in the original package in order to protect from moisture.
Do not store above 25°C.
Do not use this medicine after the expiry date (EXP) which is stated on the carton. The expiry date refers to the last day of that month.
Liquid suspension
Store in a refrigerator (2°C - 8°C). Do not freeze.
Once made up, the suspension should be used within 7 days.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Augpen ES suspension contains
The active substances are amoxicillin and clavulanic acid.
Each 5 ml of reconstituted suspension contains:
Amoxycillin Trihydrate IP equivalent to Amoxycillin 600 mg
Potassium Clavulanate Diluted IP equivalent to Clavulanic Acid 42.9 mg
Excipients q.s.
Augpen ES: A bottle of 12.5 g / 50 ml.
Revised on 16.06.2023