Azitus XL 100 and 200 Suspension
Therapy Area
Anti Infective
1.0 Generic name
Azithromycin Oral Suspension IP
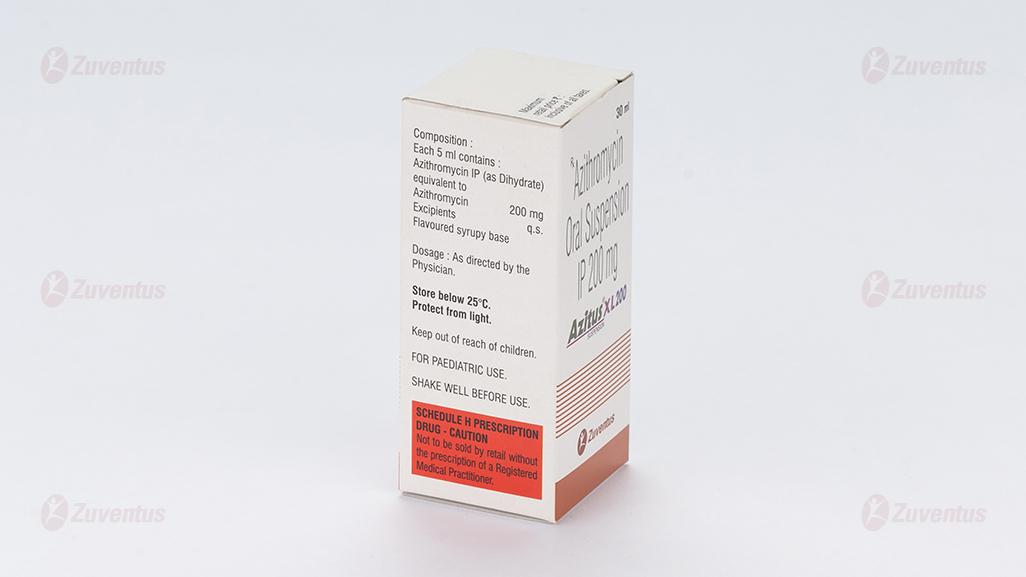
2.0 Qualitative and quantitative composition
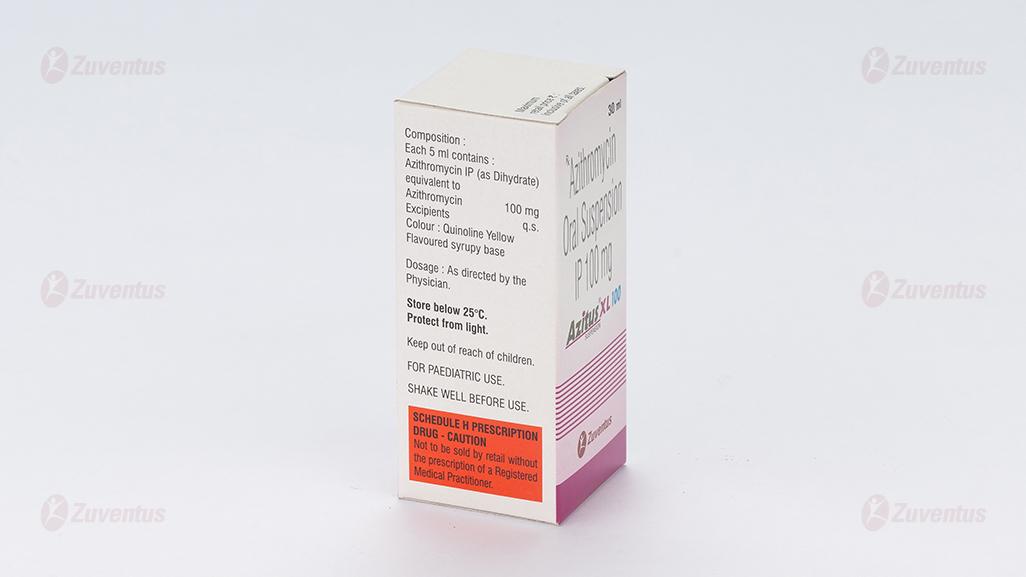
Each 5 ml contains :
Azithromycin IP (as Dihydrate)
equivalent to Azithromycin 100 mg
Excipients q.s
Colour : Quinoline Yellow
Flavoured syrupy base
Each 5 ml contains :
Azithromycin IP (as Dihydrate
equivalent to Azithromycin 200 mg
Excipients q.s.
Flavoured syrupy base
3.0 Dosage form and strength
Oral suspension 100 / 200 mg
4.0 Clinical particulars
4.1 Therapeutic indication
For susceptible infections including community acquired pneumonia, pelvic inflammatory disease.
4.2 Posology and method of administration
Children and adolescents (< 18 years)
The total dose in children aged 1 year and older is 30 mg/kg administered as 10 mg/kg once daily for three days, or over a period of five days starting with a single dose of 10 mg/kg on the first day, followed by doses of 5 mg/kg per day for the following 4 days, according to the tables shown below. There are limited data on use in children younger than 1 year.
| Weight (kg) | 3-day therapy | 5-day therapy | |
| Day 1-3 10 mg/kg/day | Day 1 10 mg/kg/day | Day 2-5 5 mg/kg/day | |
| 10 kg | 2.5 ml | 2.5 ml | 1.25 ml |
| 12 kg | 3 ml | 3 ml | 1.5 ml |
| 14 kg | 3.5 ml | 3.5 ml | 1.75 ml |
| 16 kg | 4 ml | 4 ml | 2 ml |
| 17 – 25 kg | 5 ml | 5 ml | 2.5 ml |
| 26 – 35 kg | 7.5 ml | 7.5 ml | 3.75 ml |
| 36 – 45 kg | 10 ml | 10 ml | 5 ml |
| > 45 kg | 12.5 ml | 12.5 ml | 6.25 ml |
The dose for the treatment of pharyngitis caused by Streptococcus pyogenes is an exception : in the treatment of pharyngitis caused by Streptococcus pyogenes Azithromycin has proved to be effective when it is administered to children as a single dose of 10 mg/kg or 20 mg/kg for 3 days with a maximum daily dose of 500 mg. At these two doses a comparable clinical effect was observed, even if the eradication of the bacteria was more significant at a daily dose of 20 mg/kg. Penicillin is however the drug of first choice in the treatment of pharyngitis caused by Streptococcus pyogenes and the prevention of subsequent rheumatic fever
Patients with renal impairment :
No dose adjustment is necessary in patients with mild to moderate renal impairment (GFR 10-80 ml/min).
Patients with hepatic impairment :
A dose adjustment is not necessary for patients with mild to moderately impaired liver function
Method of administration
After taking the suspension a bitter after-taste can be avoided by drinking fruit juice directly after swallowing. Azithromycin oral suspension should be given in a single daily dose. The suspension may be taken together with food.
4.3 Contraindications
The use of this product is contraindicated in patients with hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide antibiotic, or to any of the excipients listed in the formulation.
4.4 Special warnings and precautions for use Hypersensitivity
As with erythromycin and other macrolides, rare serious allergic reactions, including angioneurotic oedema and anaphylaxis (rarely fatal), dermatologic reactions including acute generalised exanthematous pustulosis (AGEP), Stevens Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) (rarely fatal) and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported. Some of these reactions with azithromycin have resulted in recurrent symptoms and required a longer period of observation and treatment. If an allergic reaction occurs, the medicinal product should be discontinued and appropriate therapy should be instituted. Physicians should be aware that reappearance of the allergic symptoms may occur when symptomatic therapy is discontinued.
Since liver is the principal route of elimination for azithromycin, the use of azithromycin should be undertaken with caution in patients with significant hepatic disease. Cases of fulminant hepatitis potentially leading to life-threatening liver failure have been reported with azithromycin. Some patients may have had pre-existing hepatic disease or may have been taking other hepatotoxic medicinal products. In case of signs and symptoms of liver dysfunction, such as rapid developing asthenia associated with jaundice, dark urine, bleeding tendency or hepatic encephalopathy, liver function tests / investigations should be performed immediately. Azithromycin administration should be stopped if liver dysfunction has emerged. In patients receiving ergot derivatives, ergotism has been precipitated by coadministration of some macrolide antibiotics. There are no data concerning the possibility of an interaction between ergot and azithromycin. However, because of the theoretical possibility of ergotism, azithromycin and ergot derivatives should not be coadministered. As with any antibiotic preparation, observation for signs of superinfection with non- susceptible organisms, including fungi is recommended.
Clostridium difficile associated diarrhoea (CDAD) has been reported with the use of nearly all antibacterial agents, including azithromycin, and may range in severity from mild diarrhoea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhoea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. In patients with severe renal impairment (GFR <10 ml/min) a 33% increase in systemic exposure to azithromycin was observed.
Cardiovascular Events
Prolonged cardiac repolarization and QT interval, imparting a risk of developing cardiac arrhythmia and torsades de pointes, have been seen in treatment with macrolides including azithromycin. Therefore as the following situations may lead to an increased risk for ventricular arrhythmias (including torsade de pointes) which can lead to cardiac arrest, azithromycin should be used with caution in patients with ongoing proarrhythmic conditions (especially women and elderly patients) such as patients :
With congenital or documented QT prolongation
- Currently receiving treatment with other active substances known to prolong QT interval such as antiarrhythmics of class IA (quinidine and procainamide ) and class III (dofetilide, amiodarone and sotalol), cisapride and terfenadine; antipsychotic agents such as pimozide; antidepressants such as citalopram; and fluoroquinolones such as moxifloxacin and levofloxacin
- With electrolyte disturbance, particularly in cases of hypokalaemia and hypomagnesemia
- With clinically relevant bradycardia, cardiac arrhythmia or severe cardiac insufficiency
Epidemiological studies investigating the risk of adverse cardiovascular outcomes with macrolides have shown variable results. Some observational studies have identified a rare short term risk of arrhythmia, myocardial infarction and cardiovascular mortality associated with macrolides including azithromycin. Consideration of these findings should be balanced with treatment benefits when prescribing azithromycin. Exacerbations of the symptoms of myasthenia gravis and new onset of myasthenia syndrome have been reported in patients receiving azithromycin therapy. Safety and efficacy for the prevention or treatment of Mycobacterium aviumcomplex in children have not been established.
The following should be considered before prescribing azithromycin :
Azithromycin oral suspension is not suitable for treatment of severe infections where a high concentration of the antibiotic in the blood is rapidly needed.
Azithromycin is not the first choice for the empiric treatment of infections in areas where the prevalence of resistant isolates is 10% or more.
In areas with a high incidence of erythromycin A resistance, it is especially important to take into consideration the evolution of the pattern of susceptibility to azithromycin and other antibiotics.
As for other macrolides, high resistance rates of Streptococcus pneumoniae (> 30 %) have been reported for azithromycin in some European countries. This should be taken into account when treating infections caused by Streptococcus pneumoniae.
Pharyngitis / tonsilitis
Azithromycin is not the substance of first choice for the treatment of pharyngitis and tonsillitis caused by Streptococcus pyogenes. For this and for the prophylaxis of acute rheumatic fever penicillin is the treatment of first choice.
Sinusitis
Often, azithromycin is not the substance of first choice for the treatment of sinusitis
Acute otitis media
Often, azithromycin is not the substance of first choice for the treatment of acute otitis media.
Skin and soft tissue infections
The main causative agent of soft tissue infections, Staphylococcus aureus, is frequently resistant to azithromycin. Therefore, susceptibility testing is considered a precondition for treatment of soft tissue infections with azithromycin.
Infected burn wounds
Azithromycin is not indicated for the treatment of infected burn wounds
Sexually transmitted disease
In case of sexually transmitted diseases a concomitant infection by T. pallidum should be excluded.
Neurological or psychiatric diseases
Azithromycin should be used with caution in patients with neurological or psychiatric disorders.
Neurological or psychiatric diseases
Azithromycin should be used with caution in patients with neurological or psychiatric disorders.
4.5 Drugs interactions
Antacids
In a pharmacokinetic study investigating the effects of simultaneous administration of antacid with azithromycin, no effect on overall bioavailability was seen although peak serum concentrations were reduced by approximately 25%. In patients receiving both azithromycin and antacids, the medicinal products should not be taken simultaneously.
Cetirizine
In healthy volunteers, coadministration of a 5-day regimen of azithromycin with cetirizine 20 mg at steady-state resulted in no pharmacokinetic interaction and no significant changes in the QT interval.
Didanosine (Dideoxyinosine)
Coadministration of 1200 mg/day azithromycin with 400 mg/day didanosine in 6 HIV-positive subjects did not appear to affect the steady-state pharmacokinetics of didanosine as compared with placebo.
Digoxin and colchicine (P-gp substrates)
Concomitant administration of macrolide antibiotics, including azithromycin, with P-glycoprotein substrates such as digoxin and colchicine, has been reported to result in increased serum levels of the P-glycoprotein substrate. Therefore, if azithromycin and P-gp substrates such as digoxin are administered concomitantly, the possibility of elevated serum concentrations of the substrate should be considered
Zidovudine
Single 1000 mg doses and multiple 1200 mg or 600 mg doses of azithromycin had little effect on the plasma pharmacokinetics or urinary excretion of zidovudine or its glucuronide metabolite. However, administration of azithromycin increased the concentrations of phosphorylated zidovudine, the clinically active metabolite, in peripheral blood mononuclear cells. The clinical significance of this finding is unclear, but it may be of benefit to patients. Azithromycin does not interact significantly with the hepatic cytochrome P450 system. It is not believed to undergo the pharmacokinetic drug interactions as seen with erythromycin and other macrolides. Hepatic cytochrome P450 induction or inactivation via cytochrome-metabolite complex does not occur with azithromycin
Ergot
Due to the theoretical possibility of ergotism, the concurrent use of azithromycin with ergot derivatives is not recommended. Pharmacokinetic studies have been conducted between azithromycin and the following drugs known to undergo significant cytochrome P450 mediated metabolism. Ergotamine derivatives : Due to the theoretical possibility of ergotism, the concurrent use of azithromycin with ergot derivatives is not recommended. Astemizole, alfentanil There are no known data on interactions with astemizole or alfentanil. Caution is advised in the co-administration of these medicines with Azithromycin because of the known enhancing effect of these medicines when used concurrently with the macrolid antibiotic erythromycin.
Atorvastatin
Co-administration of atorvastatin (10 mg daily) and azithromycin (500 mg daily) did not alter the plasma concentrations of atorvastatin (based on a HMG CoA-reductase inhibition assay). However, post-marketing cases of rhabdomyolysis in patients receiving azithromycin with statins have been reported.
Carbamazepine
In a pharmacokinetic interaction study in healthy volunteers, no significant effect was observed on the plasma levels of carbamazepine or its active metabolite in patients receiving concomitant azithromycin.
Cisapride
Cisapride is metabolized in the liver by the enzyme CYP 3A4. Because macrolides inhibit this enzyme, concomitant administration of cisapride may cause the increase of QT interval prolongation, ventricular arrhythmias and torsades de pointes
Cimetidine
In a pharmacokinetic study investigating the effects of a single dose of cimetidine, given 2 hours before azithromycin, on the pharmacokinetics of azithromycin, no alteration of azithromycin pharmacokinetics was seen.
Coumarin-type oral anticoagulants
In a pharmacokinetic interaction study, azithromycin did not alter the anticoagulant effect of a single 15 mg dose of warfarin administered to healthy volunteers. There have been reports received in the post-marketing period of potentiated anticoagulation subsequent to co-administration of azithromycin and coumarin-type oral anticoagulants. Although a causal relationship has not been established, consideration should be given to the frequency of monitoring prothrombin time when azithromycin is used in patients receiving coumarin-type oral anticoagulants.
Cyclosporin
In a pharmacokinetic study with healthy volunteers that were administered a 500 mg/day oral dose of azithromycin for 3 days and were then administered a single 10 mg/kg oral dose of cyclosporin, the resulting cyclosporin Cmax and AUC0-5 were found to be significantly elevated. Consequently, caution should be exercised before considering concurrent administration of these drugs. If co-administration of these drugs is necessary, cyclosporin levels should be monitored and the dose adjusted accordingly.
Efavirenz
Co-administration of a 600 mg single dose of azithromycin and 400 mg efavirenz daily for 7 days did not result in any clinically significant pharmacokinetic interactions.
Fluconazole
Co-administration of a single dose of 1200 mg azithromycin did not alter the pharmacokinetics of a single dose of 800 mg fluconazole. Total exposure and half-life of azithromycin were unchanged by the co-administration of fluconazole, however, a clinically insignificant decrease in Cmax (18%) of azithromycin was observed.
Indinavir
Co-administration of a single dose of 1200 mg azithromycin had no statistically significant effect on the pharmacokinetics of indinavir administered as 800 mg three times daily for 5 days.
Methylprednisolone
In a pharmacokinetic interaction study in healthy volunteers, azithromycin had no significant effect on the pharmacokinetics of methylprednisolone.
Midazolam
In healthy volunteers, co-administration of azithromycin 500 mg/day for 3 days did not cause clinically significant changes in the pharmacokinetics and pharmacodynamics of a single 15 mg dose of midazolam.
Nelfinavir
Co-administration of azithromycin (1200 mg) and nelfinavir at steady state (750 mg three times daily) resulted in increased azithromycin concentrations. No clinically significant adverse effects were observed and no dose adjustment is required
Rifabutin
Co-administration of azithromycin and rifabutin did not affect the serum concentrations of either medicinal product. Neutropenia was observed in subjects receiving concomitant treatment of azithromycin and rifabutin. Although neutropenia has been associated with the use of rifabutin, a causal relationship to combination with azithromycin has not been established.
Sildenafil
In normal healthy male volunteers, there was no evidence of an effect of azithromycin (500 mg daily for 3 days) on the AUC and Cmax of sildenafil or its major circulating metabolite.
Terfenadine
Pharmacokinetic studies have reported no evidence of an interaction between azithromycin and terfenadine. There have been rare cases reported where the possibility of such an interaction could not be entirely excluded; however there was no specific evidence that such an interaction had occurred.
Theophylline
There is no evidence of a clinically significant pharmacokinetic interaction when azithromycin and theophylline are co-administered to healthy volunteers.
Triazolam
In 14 healthy volunteers, co-administration of azithromycin 500 mg on Day 1 and 250 mg on Day 2 with 0.125 mg triazolam on Day 2 had no significant effect on any of the pharmacokinetic variables for triazolam compared to triazolam and placebo.
Trimethoprim / sulfamethoxazole
Co-administration of trimethoprim/sulfamethoxazole (160 mg/800 mg) for 7 days with azithromycin 1200 mg on Day 7 had no significant effect on peak concentrations, total exposure or urinary excretion of either trimethoprim or sulfamethoxazole. Azithromycin serum concentrations were similar to those seen in other studies. Substances that prolong the QT interval Azithromycin should not be used concomitantly with other active substances that prolong the QT interval
4.6 Use in special populations
Pregnancy
There are no adequate data from the use of Azithromycin in pregnant women. In reproduction toxicity studies in animals azithromycin was shown to pass the placenta, but no teratogenic effects were observed. The safety of azithromycin has not been confirmed with regard to the use of the active substance during pregnancy. Therefore Azithromycin should only be used during pregnancy if the benefit outweighs the risk.
Breastfeeding
Azithromycin has been reported to be secreted into human breast milk, but there are no adequate and well-controlled clinical studies in breastfeeding women that have characterized the pharmacokinetics of azithromycin excretion into human breast milk.
Fertility
In fertility studies conducted in rat, reduced pregnancy rates were noted following administration of azithromycin. The relevance of this finding to humans is unknown.
4.7 Effects on ability to drive and use machines
There is no evidence to suggest that azithromycin may have an effect on a patient's ability to drive or operate machinery. Visual impairment and vision blurred may have an effect on a patient's ability to drive or operate machinery.
4.8 Undesirable effects
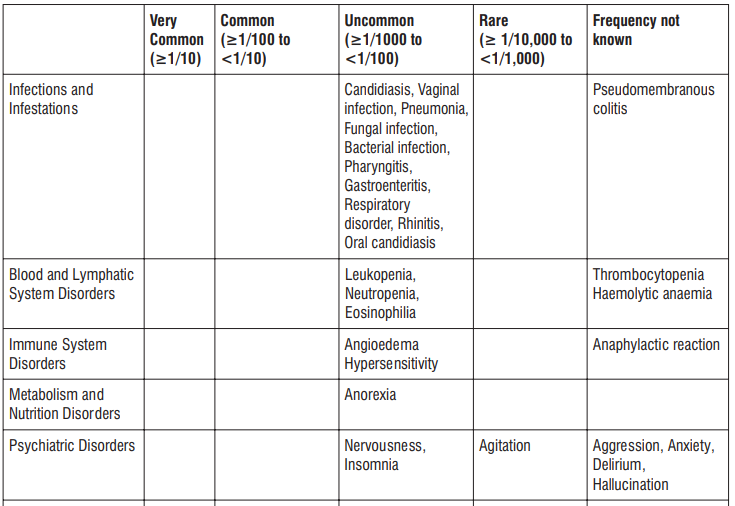
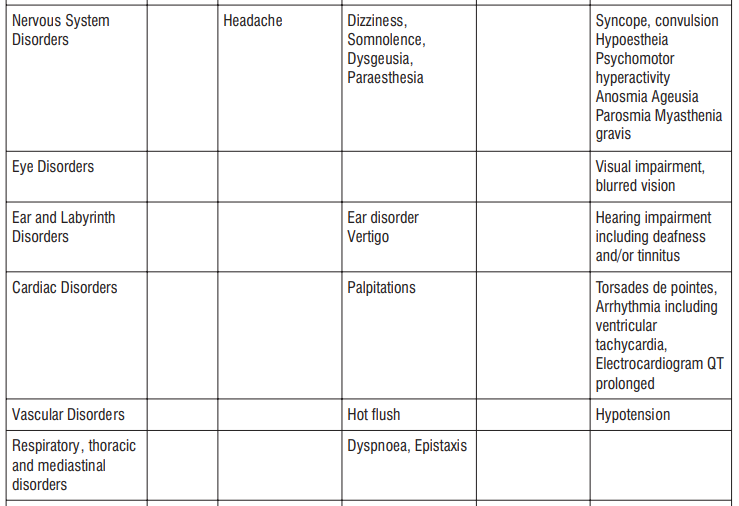
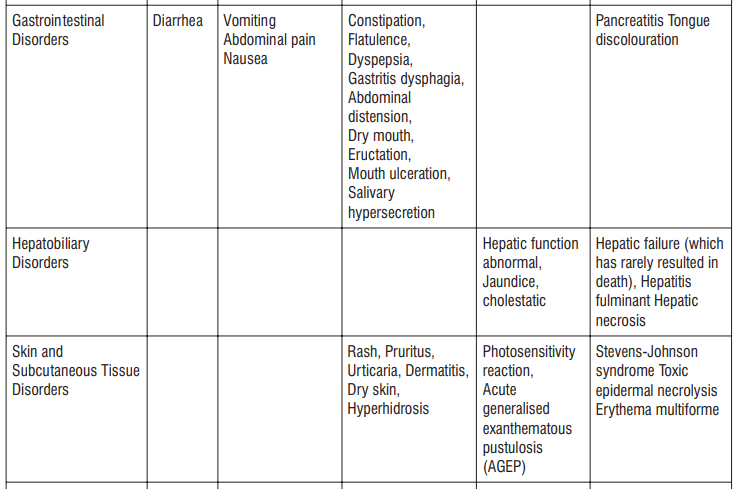
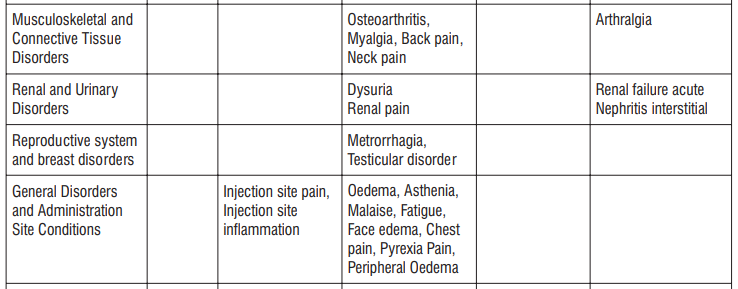
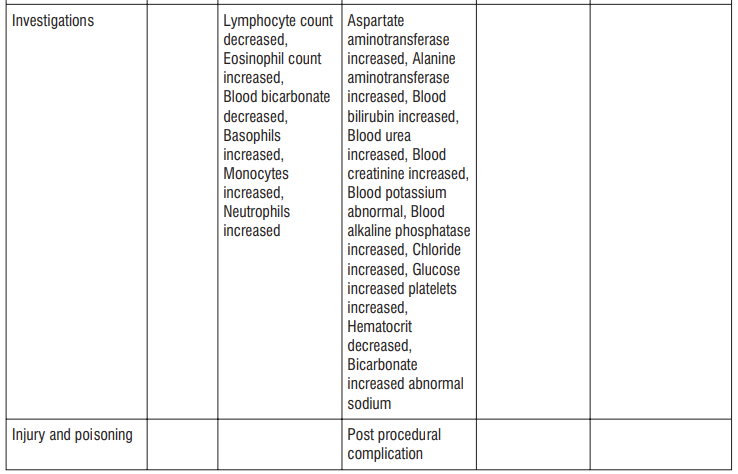
Adverse reactions possibly or probably related to Mycobacterium Avium Complex prophylaxis and treatment base Adverse reactions possibly or probably related to Mycobacterium Avium Complex prophylaxis and treatment based on clinical trial experience and post-marketing surveillance. These adverse reactions differ from those reported with immediate release or the prolonged release formulations, either in kind or in frequency :
Reporting of suspected adverse reactions
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
4.9 Overdose
Adverse events experienced in higher than recommended doses were similar to those seen at normal doses. In the event of overdosage, general symptomatic and supportive measures are indicated as required.
5.0 Pharmacological properties
5.1 Mechanism of action
Azithromycin is an azalide, a sub-class of the macrolide antibiotics. By binding to the 50S-ribosomal sub-unit, azithromycin avoids the translocation of peptide chains from one side of the ribosome to the other. As a consequence of this, RNAdependent protein synthesis in sensitive organisms is prevented.
5.2 Pharmacodynamic properties
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
Table : Antibacterial spectrum of Azithromycin
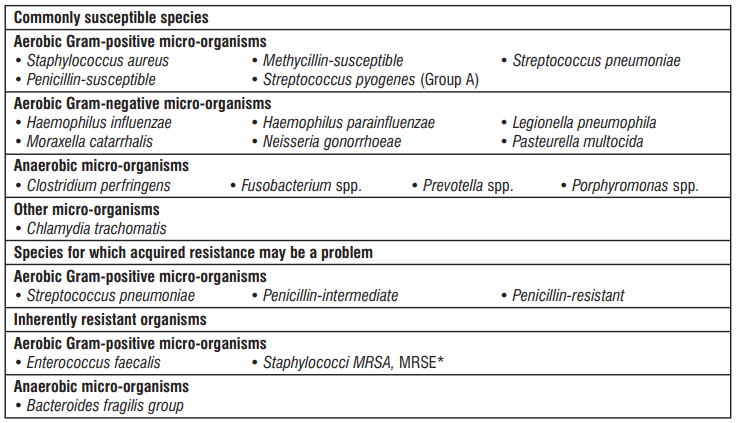
* Methycillin-resistant staphylococci have a very high prevalence of acquired resistance to macrolides and have been placed here because they are rarely susceptible to azithromycin.
5.3 Pharmacokinetic properties
Absorption
The biological availability of azithromycin after oral administration is approximately 37%. Peak plasma levels are achieved 2-3 hours after taking the medicinal product.
Distribution
After oral administration, azithromycin is distributed throughout the entire body. Pharmacokinetic studies have shown clearly higher azithromycin levels in the tissues than in the plasma (up to 50 times the maximum observed concentration in plasma). This indicates that the substance is bound in the tissues in considerable quantities. Concentrations in the infected tissues, such as lungs, tonsil and prostate are higher than the MRC90 of the most frequently occurring pathogens after a single dose of 500 mg. The protein binding of azithromycin in serum is variable and varies, depending on the serum concentration, from 52% at 0.05 mg/l to 12% at 0.5 mg/l. The steady state distribution volume is 31.1 l/kg.
Elimination
The terminal plasma-elimination half-life closely follows the tissue depletion half-life from 2 to 4 days. Approximately 12% of an intravenously administered dose of azithromycin is, over a period of 3 days, excreted unchanged in the urine. High concentrations of unchanged azithromycin were found in human bile. In this, ten metabolites were also detected (formed by N- and O- desmethylation, by hydroxylation of the desosamin and aglycon rings and by splitting the cladinose conjugate). A comparison of fluid chromatography and microbiological assessment methods shows that the metabolites are microbiologically inactive.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
In animal tests in which the doses used amounted to 40 times the clinical therapeutic doses, azithromycin was found to have caused reversible phospholipidosis, but as a rule no true toxicological consequences were observed which were associated with this. The relevance of this finding to humans receiving azithromycin in accordance with the recommendations is unknown. Electrophysiological investigations have shown that azithromycin prolongs the QT interval. Mutagenic potential : There was no evidence of a potential for genetic and chromosome mutations in in-vivo and in-vitro test models. Reproductive toxicity : In embryotoxicity studies in mice and rats no teratogenic effects were observed. In rats, azithromycin dosages of 100 and 200 mg/kg bodyweight/day led to slight retardations in fetal ossification and in maternal weight gain. In peri-/postnatal studies in rats, slight retardations in physical development and delay in reflex development were observed following treatment with 50 mg/kg/day azithromycin and above.
7.0 Description
Azithromycin suspension contains the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the
Chemical name : (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribohexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)- βD-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one.
Molecular formula : C38H72N2O12
Molecular weight : 749.0 g/mol
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
30 Months
8.3 Packaging information
A bottle of 30 ml.
8.4 Storage and handing instructions
Store below 25°C. Protect from light.
Keep out of reach of children.
9.0 Patient counselling information
- Azithromycin suspension may be taken with or without food. However, increased tolerability has been observed when tablets are taken with food.
- Patients should also be cautioned not to take aluminum- and magnesium-containing antacids and azithromycin simultaneously.
- The patient should be directed to discontinue azithromycin immediately and contact a physician if any signs of an allergic reaction occur.
- Patients should be counselled that antibacterial drugs, including azithromycin, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When azithromycin is prescribed to treat bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by azithromycin or other antibacterial drugs in the future
- Diarrhea is a common problem caused by antibacterial which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial. If this occurs, patients should contact their physician as soon as possible.
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you:
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What AZITUS® XL is and what it is used for
2. What you need to know before you take AZITUS® XL
3. How to take AZITUS® XL
4. Possible side effects
5. How to store AZITUS® XL
6. Contents of the pack and other information
1. What AZITUS® XL is and what it is used for
This medicine contains azithromycin, which is one of a group of antibiotics called macrolides. It is used to treat infections caused by certain bacteria and other micro-organisms which include:
- Susceptible infections including community-acquired pneumonia, pelvic inflammatory disease.
You must talk to a doctor if you do not feel better or if you feel worse.
2. What you need to know before you take AZITUS® XL
Do not take AZITUS® XL:
- if you/your child are allergic to azithromycin or any other macrolide antibiotic such as erythromycin or clarithromycin or any of the ingredients. An allergic reaction may cause skin rash or wheezing.
Warnings and precautions
Talk to your doctor or pharmacist before taking AZITUS® XL if you/your child have or have had
any of the following:
- kidney problems
- heart conditions
- diabetes
- liver problems: your doctor may need to monitor your liver function or stop the treatment
- myasthenia gravis (a condition that causes certain muscles to become weak)
- or if you are taking any ergot derivatives such as ergotamine (used to treat migraine) as these medicines should not be taken together with AZITUS® XL.
Tell your doctor immediately if you feel your heart beating in your chest or have an abnormal heartbeat, or get dizzy or faint or suffer from any muscle weakness when taking AZITUS® XL.
If you develop diarrhoea or loose stools during or after treatment, tell your doctor at once. Do not take any medicine to treat your diarrhoea without first checking with your doctor. If your diarrhoea continues, please inform your doctor.
Other medicines and AZITUS® XL
Tell your doctor or pharmacist if you/your child are taking, have recently taken or might take any other medicines.
In particular, AZITUS® XL may interact with the medicines listed below:
- ergot or ergotamine, see ‘Warnings and precautions’ section
- warfarin or any similar medicine to prevent blood clots
- ciclosporin (used to suppress the immune system to prevent and treat rejection of a transplanted organ or bone marrow)
- antacids (for indigestion)
- digoxin (used to treat heart failure)
- colchicine (used for gout and familial Mediterranean fever)
- terfenadine (for hay fever or a skin allergy).
Pregnancy, breast-feeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. There is insufficient information available about the use of Azithromycin during pregnancy. Therefore, you should not use Azithromycin during pregnancy, unless explicitly advised by your doctor. Azithromycin is partially passed through the mother’s milk; it is not known whether azithromycin may have adverse effects on the breastfed infant. Breastfeeding should therefore be discontinued during treatment with Azithromycin. It is recommended to discard the milk during treatment and up until 2 days after discontinuation of treatment. Breastfeeding may be resumed thereafter.
Driving and using machines
There are no data available about the influence of Azithromycin on the ability to drive or operate machines. However, AZITUS® XL may cause dizziness and seizures so make sure you are not affected before driving or operating machinery
3. How to take AZITUS® XL
Always take or give this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
AZITUS® XL suspension is generally used for children under 45 kg. It may also be used in adults and older children who have difficulty swallowing capsules.
AZITUS® XL suspension is not affected by food or drink.
Children under 45 kg
The recommended dose in children is 10 mg for each kg of body weight, given as a single daily dose for 3-5 days.
Adults and children over 45 kg
The recommended dose in adults and in children over 45 kg is 500 mg taken as a single dose, for 3-5 days. For some diseases such as Chlamydia, the recommended dose is 1 g daily taken as a single dose. For gonorrhoea, the recommended dose is 1 g or 2 g of azithromycin in combination with 250 or 500 mg of ceftriaxone.
You should tell your doctor if you/your child have kidney or liver problems as your doctor may need to alter the normal dose.
Doctors sometimes prescribe different doses to the recommended dose. The label on the pack will tell you which dose you/your child should take. If you are still not sure, ask your doctor or pharmacist.
Always continue with the course of treatment even if you/your child feel better. If your infection gets worse or you do not start to feel better within a few days or a new infection develops, go back and see your doctor.
How to give AZITUS® XL Suspension to children less than 3 years of age
If your child is under three years of age or weighs up to 15 kg in bodyweight, you should measure the dose as clearly as possible using the 5 ml oral dosing plastic cup provided. The plastic cup is graduated in 2.5 ml divisions, providing 10 mg of azithromycin (the active ingredient) in every graduation.
Filling the plastic cup with medicine
1.Shake the bottle before use and remove the cap.
2.Check the dispensing label attached by your pharmacist to see how much medicine needs to be taken.
3.Turn the bottle upside down slowly while holding the graduated plastic cup in place.
4.Slowly pour the suspension into a plastic cup so that the top edge is level with the graduation mark corresponding to the quantity in the millilitres (ml) prescribed by your doctor.
5.If large bubbles can be seen in the plastic cup, slowly push back the medicine back into the bottle. Repeat step 4 again.
6.Hold the plastic cup and bottle firmly. Turn the bottle upright, with the plastic cap still in place.
Giving the medicine using the plastic cup
1.Make sure your child is supported in an upright position.
2.Put the upper end of the plastic cup carefully into your child’s mouth. Point the upper end of the plastic cup towards the inside of your child’s cheek.
3.Allow your child some time to swallow the medicine.
4.Replace the child-proof cap on the bottle. Wash the plastic cup as instructed below.
5.Where daily doses of less than 5ml have been given for three days, some suspension will remain in the bottle. This remaining suspension should be discarded.
How to give AZITUS® XL Suspension to children between 3 and 18 years of age
|
Bodyweight and age |
Dose and duration |
|
15-25 kg bodyweight (3-7 years): |
3.5 to 5 ml (200 mg) once daily for 3-5 days. |
|
26-35 kg bodyweight (8-11 years): |
7.5 ml (300 mg), once daily for 3-5 days. |
|
36-45 kg bodyweight (12-14 years): |
10 ml (400 mg), once daily for 3-5 days. |
Warning: if giving this medicine to a child, ensure that while receiving the medicine he/she is supported in an upright position to avoid the risk of choking.
If you/your child takes more AZITUS® XL than you should
If you/your child takes too much AZITUS® XL they may feel unwell. Tell your doctor or contact your nearest hospital casualty department immediately. Take any remaining medicine with you.
If you forget to take or give AZITUS® XL
If you forget to take AZITUS® XL take it as soon as you can. Take your next dose at the right time. Do not take a double dose to make up for a forgotten dose.
If you stop taking AZITUS® XL
If you/your child stop taking AZITUS® XL too soon, the infection may return. Take AZITUS® XL for the full-time of treatment, even when you/your child begin to feel better. If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects although not everybody gets them.
Tell your doctor immediately if you experience any of the following symptoms after taking this medicine as the symptoms can be severe.
- sudden wheeziness, difficulty in breathing, swelling of eyelids, face or lips, rash or itching (especially affecting the whole body)
- severe or prolonged diarrhoea, which may have blood or mucus in it, during or after treatment with AZITUS® XL as this may be a sign of serious bowel inflammation
- severe skin rash causing redness and flaking
- rapid or irregular heartbeat
- low blood pressure
- Serious skin reactions:
- blistering of the skin, mouth, eyes and genitals (Stevens-Johnson Syndrome (SJS))
- blistering of the skin, severe skin reaction (Toxic Epidermal Necrosis (TEN))
- skin rash accompanied by other symptoms such as fever, swollen glands and an increase of eosinophils (a type of white blood cell). A rash appears as small, itchy red bumps (Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS))
- skin eruption that is characterised by the rapid appearance of areas of red skin studded with small pustules (small blisters filled with white/yellow fluid) (Acute Generalized Exanthematous Pustulosis (AGEP)).
Stop taking azithromycin if you develop these skin symptoms and contact your doctor or seek medical attention immediately.
The most common side effects that occur when taking AZITUS® XL are listed below. These may go away during treatment as your body adjusts to the medicine. Tell your doctor if any of these side effects continue to bother you:
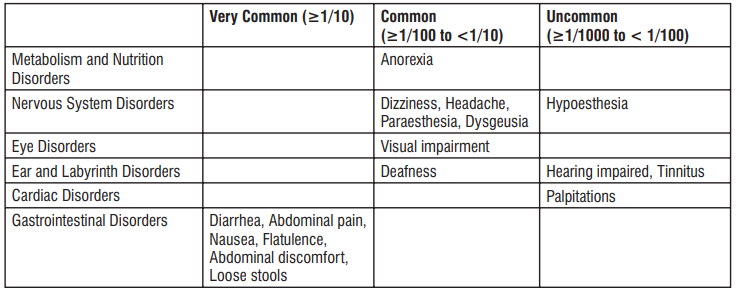
Very common: may affect more than 1 in 10 people
• stomach cramps, feeling sick, diarrhoea, wind.
Common: may affect up to 1 in 10 people
•dizziness, headache
•numbness or pins and needles
•being sick, indigestion
•loss of appetite, taste disturbance
•visual disturbances, deafness
•skin rash and /or itching
•joint pain
•low numbers of lymphocytes (a type of white blood cell), higher number of eosinophils (type of white blood cells)
•low blood bicarbonate
•tiredness or weakness.
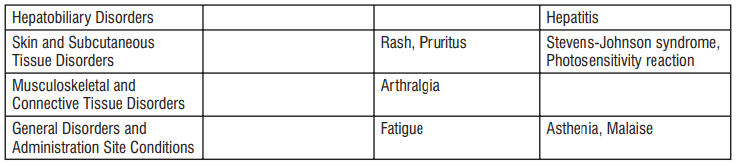
Uncommon: may affect up to 1 in 100 people
•yeast infections of the mouth and vagina (thrush)
•low numbers of leukocytes (a type of white blood cell), low number of neutrophils (a type of white blood cell)
•allergic reactions of various severity
•skin more sensitive to sunlight than normal
•feeling nervous
•reduced sense of touch or sensation (hypoesthesia)
•sleepiness or sleeplessness (insomnia)
•poor hearing or ringing in the ears
•heart palpitations, chest pain
•constipation, stomach pain associated with diarrhoea and fever
•inflammation of the liver (hepatitis), changes in liver enzymes.
•general loss of strength
•swelling
•general discomfort
•abnormal laboratory test values (e.g. blood or liver tests).
Rare: may affect up to 1 in 1,000 people
•agitation
•vertigo
•changes in liver function
Not known: frequency cannot be estimated from the available data
•fits or fainting
•aggression or anxiety
•feeling hyperactive
•localised muscle weakness
•loss of smell or altered sense of smell, loss of taste
•tongue discolouration
•inflammation of the pancreas (pancreatitis)
•inflammation of the kidney or kidney failure
•yellowing of the skin or eyes (jaundice) or liver failure (rarely life-threatening)
•bruising or prolonged bleeding after injury
•abnormal electrocardiogram (ECG)
•reduction in red blood cells which can make the skin pale and cause weakness or breathlessness.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store AZITUS® XL
Keep this medicine out of the sight and reach of children.
Do not take or give this medicine after the expiry date which is stated on the bottle. The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions. Any unused medicine should be discarded after 5 days.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What AZITUS® XL contains
The active substance is azithromycin (100 or 200 mg in 5 mL).
What AZITUS® XL looks like
AZITUS® XL is a suspension with Quinoline Yellow WS color and comes in the 30 ml bottle size.
Marketing Authorisation Holder
Zuventus Healthcare Limited
Zuventus House, Plot Y2, CTS No.: 358/A2,
Near Nahur Railway Station,
Nahur (W), Mumbai, 400078 Maharashtra, India.
This leaflet was last revised in 06/2023.