Bisotrak T Tablets 2.5mg
Therapy Area
Cardiology
1.0 Generic name
Bisoprolol Fumarate and Telmisartan Tablets
2.0 Qualitative and quantitative composition
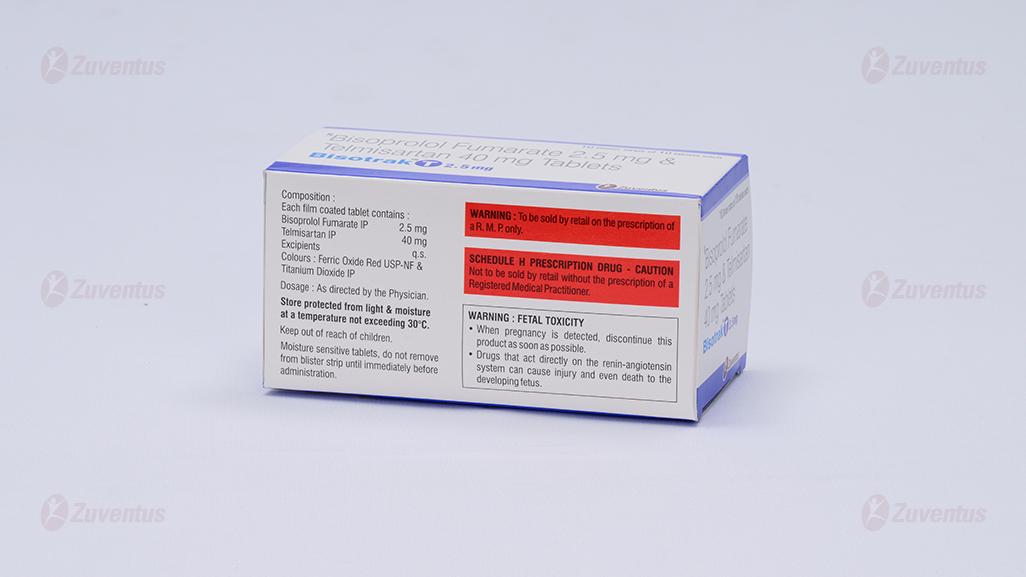
Each film coated tablet contains: Bisoprolol Fumarate IP 2.5/5 mg Telmisaratan IP 40 mg
3.0 Dosage form and strength form
Film Coated Tablet Bisoprolol Fumarate (2.5/5) mg and Telmisartan 40 mg
4.0 Clinical particulars
4.1 Therapeutic indications
For the treatment of mild to moderate hypertension.
4.2 Posology and method of administration
1 tablet once daily.
FDC of Telmisartan and Bisoprolol Fumarate Tablets dosage should be adjusted according to each patient´s need, preferably starting with lower dose and titrate to higher dose in cases where the target blood pressure is not achieved, the dose of Telmisartan can be increased to a maximum of 80 mg and accordingly Bisoprolol Fumarate10 mg once daily.
4.3 Contraindications
- Hypersensitivity to the bisoprolol, telmisartan or to any of the excipients listed in the formulation
- Acute heart failure or during episodes of heart failure decompensation requiring i.v. inotropic therapy
- Cardiogenic shock
- Second or third degree AV block (without a pacemaker)
- Sick sinus syndrome
- Sinoatrial block
- Symptomatic bradycardia
- Symptomatic hypotension
- Severe bronchial asthma or severe chronic obstructive pulmonary disease
- Severe forms of peripheral arterial occlusive disease or severe forms of Raynaud's syndrome
- Untreated phaeochromocytoma
- Metabolic acidosis
- Second and third trimester of pregnancy
- Biliary obstructive disorders since telmisartan can induce severe hypotension and renal insufficiency
- Severe hepatic impairment.
- The concomitant use of telmisartan with aliskiren-containing products is contraindicated in patients with diabetes mellitus or renal impairment (GFR < 60 ml/min/1.73 m2)
4.4 Special warnings and precautions for use
Bisoprolol Fumarate
The treatment of stable chronic heart failure with bisoprolol has to be initiated with a special titration phase.
In patients with ischaemic heart disease the cessation of therapy with bisoprolol must not be done abruptly unless clearly indicated, because this may lead to transitional worsening of heart condition.
Precautions
In hypertension or angina pectoris
Bisoprolol must be used with caution in patients with hypertension or angina pectoris and accompanying heart failure.
In chronic heart failure
The initiation of treatment of stable chronic heart failure with bisoprolol necessitates regular monitoring.
There is no therapeutic experience of bisoprolol treatment of heart failure in patients with the following diseases and conditions:
- insulin dependent diabetes mellitus (type I)
- severely impaired renal function
- severely impaired hepatic function
- restrictive cardiomyopathy
- congenital heart disease
- haemodynamically significant organic valvular disease
- myocardial infarction within 3 months
Bisoprolol must be used with caution in:
- bronchospasm (bronchial asthma, obstructive airways diseases). In bronchial asthma or other chronic obstructive lung diseases, which may cause symptoms, bronchodilating therapy is recommended to be given concomitantly. Occasionally an increase of the airway resistance may occur in patients with asthma, therefore the dose of beta2-stimulants may have to be increased.
- diabetes mellitus with large fluctuations in blood glucose values; symptoms of hypoglycaemia (e.g. tachycardia, palpitations or sweating) can be masked
- strict fasting
- ongoing desensitisation therapy. As with other beta-blockers, bisoprolol may increase both the sensitivity towards allergens and the severity of anaphylactic reactions. Epinephrine treatment does not always yield the expected therapeutic effect.
- First degree AV block
- Prinzmetal's angina; Cases of coronary vasospasm have been observed. Despite its high beta1-selectivity, angina attacks cannot be completely excluded when bisoprolol is administered to patients with Prinzmetal's angina.
- peripheral arterial occlusive disease. Aggravation of symptoms may occur especially when starting therapy.
- general anesthesia
In patients undergoing general anaesthesia beta-blockade reduces the incidence of arrhythmias and myocardial ischemia during induction and intubation and the post-operative period. It is currently recommended that maintenance beta-blockade be continued peri-operatively. The anesthetist must be aware of beta-blockade because of the potential for interactions with other drugs, resulting in bradyarrhythmias, attenuation of the reflex tachycardia and the decreased reflex ability to compensate for blood loss. If it is thought necessary to withdraw beta-blocker therapy before surgery, this should be done gradually and completed about 48 hours before anaesthesia.
Combination of bisoprolol with calcium antagonists of the verapamil or diltiazem type, with Class I antiarrhythmic drugs and with centrally acting antihypertensive drugs is generally not recommended.
Although cardioselective (beta1) beta-blockers may have less effect on lung function than non-selective beta-blockers, as with all beta-blockers, these should be avoided in patients with obstructive airways diseases, unless there are compelling clinical reasons for their use. Where such reasons exist, bisoprolol may be used with caution. In patients with obstructive airways diseases, the treatment with bisoprolol should be started at the lowest possible dose and patients should be carefully monitored for new symptoms (e.g. dyspnea, exercise intolerance, cough). In bronchial asthma or other chronic obstructive lung diseases, which may cause symptoms, bronchodilating therapy should be given concomitantly. Occasionally an increase of the airway resistance may occur in patients with asthma, therefore the dose of beta2-stimulants may have to be increased.
Patients with psoriasis or with a history of psoriasis should only be given beta-blockers (e.g. bisoprolol) after carefully balancing the benefits against the risks. In patients with phaeochromocytoma bisoprolol must not be administered until after alpha-receptor blockade. Under treatment with bisoprolol the symptoms of a thyrotoxicosis may be masked.
Telmisartan
Hepatic impairment
Telmisartan should be used only with caution in patients with mild to moderate hepatic impairment.
Renovascular hypertension
There is an increased risk of severe hypotension and renal insufficiency when patients with bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney are treated with medicinal products that affect the renin-angiotensin-aldosterone system.
Renal impairment and kidney transplantation
When Telmisartan is used in patients with impaired renal function, periodic monitoring of potassium and creatinine serum levels is recommended. There is no experience regarding the administration of Telmisartan in patients with recent kidney transplantation
Intravascular hypovolemia
Symptomatic hypotension, especially after the first dose of Telmisartan, may occur in patients who are volume and/or sodium depleted by vigorous diuretic therapy, dietary salt restriction, diarrhea or vomiting. Such conditions should be corrected before the administration of Telmisartan. Volume and/or sodium depletion should be corrected prior to administration of Telmisartan.
Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
There is evidence that the concomitant use of ACE-inhibitors, angiotensin II receptor blockers or aliskiren increases the risk of hypotension, hyperkalaemia and decreased renal function (including acute renal failure). Dual blockade of RAAS through the combined use of ACE-inhibitors, angiotensin II receptor blockers or aliskiren is therefore not recommended.
If dual blockade therapy is considered absolutely necessary, this should only occur under specialist supervision and subject to frequent close monitoring of renal function, electrolytes and blood pressure.
ACE-inhibitors and angiotensin II receptor blockers should not be used concomitantly in patients with diabetic nephropathy.
Other conditions with stimulation of the renin-angiotensin-aldosterone system
In patients whose vascular tone and renal function depend predominantly on the activity of the renin-angiotensin-aldosterone system (e.g. patients with severe congestive heart failure or underlying renal disease, including renal artery stenosis), treatment with medicinal products that affect this system such as telmisartan has been associated with acute hypotension, hyperazotaemia, oliguria, or rarely acute renal failure.
Primary aldosteronism
Patients with primary aldosteronism generally will not respond to antihypertensive medicinal products acting through inhibition of the renin-angiotensin system. Therefore, the use of telmisartan is not recommended.
Aortic and mitral valve stenosis, obstructive hypertrophic cardiomyopathy
As with other vasodilators, special caution is indicated in patients suffering from aortic or mitral stenosis, or obstructive hypertrophic cardiomyopathy.
Diabetic patients treated with insulin or antidiabetics
In these patients hypoglycaemia may occur under telmisartan treatment. Therefore, in these patients an appropriate blood glucose monitoring should be considered; a dose adjustment of insulin or antidiabetics may be required, when indicated.
Hyperkalaemia
The use of medicinal products that affect the renin-angiotensin-aldosterone system may cause hyperkalaemia.
In the elderly, in patients with renal insufficiency, in diabetic patients, in patients concomitantly treated with other medicinal products that may increase potassium levels, and/or in patients with intercurrent events, hyperkalaemia may be fatal. Before considering the concomitant use of medicinal products that affect the renin-angiotensin-aldosterone system, the benefit risk ratio should be evaluated.
The main risk factors for hyperkalaemia to be considered are:
- Diabetes mellitus, renal impairment, age (> 70 years)
- Combination with one or more other medicinal products that affect the renin-angiotensin-aldosterone system and/or potassium supplements. Medicinal products or therapeutic classes of medicinal products that may provoke hyperkalaemia are salt substitutes containing potassium, potassium-sparing diuretics, ACE inhibitors, angiotensin II receptor antagonists, non-steroidal anti-inflammatory medicinal products (NSAIDs, including selective COX-2 inhibitors), heparin, immunosuppressives (cyclosporin or tacrolimus), and trimethoprim.
- Intercurrent events, in particular dehydration, acute cardiac decompensation, metabolic acidosis, worsening of renal function, sudden worsening of the renal condition (e.g. infectious diseases), cellular lysis (e.g. acute limb ischemia, rhabdomyolysis, extend trauma).
Close-monitoring of serum potassium in at risk patients is recommended.
Other
As with any antihypertensive agent, excessive reduction of blood pressure in patients with ischaemic cardiopathy or ischaemic cardiovascular disease could result in a myocardial infarction or stroke.
4.5 Drug interactions
Bisoprolol Fumarate
Combinations not recommended
Applies only to chronic heart failure
Class-I antiarrhythmic drugs (e.g. quinidine, disopyramide, lidocaine, phenytoin, flecainide, propafenone): effect on atrio-ventricular conduction time may be potentiated and negative inotropic effect increased.
Applies to all indications
Calcium antagonists of the verapamil type and to a lesser extent of the diltiazem type: negative effect on contractility and atrio-ventricular conduction. Intravenous administration of verapamil in patients on beta-blocker treatment may lead to profound hypotension and atrio-ventricular block.
Centrally-acting antihypertensive drugs (e.g. clonidine, methyldopa, moxonidine, rilmenidine): concomitant use of centrally-acting antihypertensive drugs may further decrease the central sympathetic tonus and may thus lead to reduction of heart rate and cardiac output and to vasodilatation. Abrupt withdrawal, particularly if prior to beta-blocker discontinuation, may increase the risk of “rebound hypertension”.
Combinations to be used with caution
Applies only to hypertension or angina pectoris
Class-I antiarrhythmic drugs (e.g. quinidine, disopyramide; lidocaine, phenytoin; flecainide propafenone): Effect on atrioventricular conduction time may be potentiated and negative inotropic effect increased.
Applies to all indications
Calcium antagonists of the dihydropyridine type such as felodipine and amlodipine: Concomitant use may increase the risk of hypotension, and an increase in the risk of a further deterioration of the ventricular pump function in patients with heart failure cannot be excluded.
Class-III antiarrhythmic drugs (e.g. amiodarone): Effect on atrio-ventricular conduction time may be potentiated. Parasympathomimetic drugs: Concomitant use may increase atrio-ventricular conduction time and the risk of bradycardia.
Topical beta-blocking agents (e.g. eye drops for glaucoma treatment) may add to the systemic effects of bisoprolol.
Insulin and oral antidiabetic drugs: Increase of blood sugar lowering effect. Blockade of beta-adrenoreceptors may mask symptoms of hypoglycaemia.
Anaesthetic agents: Attenuation of the reflex tachycardia and increase of the risk of hypotension.
Digitalis glycosides: Increase of atrio-ventricular conduction time, reduction in heart rate. Non-steroidal anti-inflammatory drugs (NSAIDs): NSAIDs may reduce the hypotensive effect of bisoprolol.
β-Sympathomimetic agents (e.g. isoprenaline, dobutamine): Combination with bisoprolol may reduce the effect of both agents.
Sympathomimetics that activate both β- and α-adrenoceptors (e.g. norepinephrine, epinephrine): Combination with bisoprolol may unmask the α-adrenoceptor-mediated vasoconstrictor effects of these agents leading to blood pressure increase and exacerbated intermittent claudication. Such interactions are considered to be more likely with nonselective β-blockers.
Concomitant use with antihypertensive agents as well as with other drugs with blood pressure lowering potential (e.g. tricyclic antidepressants, barbiturates, phenothiazines) may increase the risk of hypotension.
Combinations to be considered
Mefloquine: increased risk of bradycardia
Monoamine oxidase inhibitors (except MAO-B inhibitors): Enhanced hypotensive effect of the beta-blocking agents but also risk for hypertensive crisis.
Rifampicin: Slight reduction of the half-life of bisoprolol possible due to the induction of hepatic drug metabolising enzymes. Normally no dosage adjustment is necessary. Ergotamine derivatives: Exacerbation of peripheral circulatory disturbances.
Telmisartan
Digoxin
When telmisartan was co-administered with digoxin, median increases in digoxin peak plasma concentration (49%) and in trough concentration (20%) were observed. When initiating, adjusting, and discontinuing telmisartan, monitor digoxin levels in order to maintain levels within the therapeutic range.
As with other medicinal products acting on the renin-angiotensin-aldosterone system, telmisartan may provoke hyperkalaemia. The risk may increase in case of treatment combination with other medicinal products that may also provoke hyperkalaemia (salt substitutes containing potassium, potassium-sparing diuretics, ACE inhibitors, angiotensin II receptor antagonists, non-steroidal anti-inflammatory medicinal products (NSAIDs, including selective COX-2 inhibitors), heparin, immunosuppressives (cyclosporin or tacrolimus), and trimethoprim).
The occurrence of hyperkalaemia depends on associated risk factors. The risk is increased in case of the above-mentioned treatment combinations. The risk is particularly high in combination with potassium sparing-diuretics, and when combined with salt substitutes containing potassium. A combination with ACE inhibitors or NSAIDs, for example, presents a lesser risk provided that precautions for use are strictly followed. Concomitant use not recommended
Potassium sparing diuretics or potassium supplements
Angiotensin II receptor antagonists such as telmisartan, attenuate diuretic induced potassium loss. Potassium sparing diuretics e.g. spirinolactone, eplerenone, triamterene, or amiloride, potassium supplements, or potassium-containing salt substitutes may lead to a significant increase in serum potassium. If concomitant use is indicated because of documented hypokalaemia, they should be used with caution and with frequent monitoring of serum potassium.
Lithium
Reversible increases in serum lithium concentrations and toxicity have been reported during concomitant administration of lithium with angiotensin converting enzyme inhibitors, and with angiotensin II receptor antagonists, including telmisartan. If use of the combination proves necessary, careful monitoring of serum lithium levels is recommended. Concomitant use requiring caution
Non-steroidal anti-inflammatory medicinal products
NSAIDs (i.e. acetylsalicylic acid at anti-inflammatory dosage regimens, COX-2 inhibitors and non-selective NSAIDs) may reduce the antihypertensive effect of angiotensin II receptor antagonists. In some patients with compromised renal function (e.g. dehydrated patients or elderly patients with compromised renal function), the co-administration of angiotensin II receptor antagonists and agents that inhibit cyclo-oxygenase may result in further deterioration of renal function, including possible acute renal failure, which is usually reversible. Therefore, the combination should be administered with caution, especially in the elderly. Patients should be adequately hydrated and consideration should be given to monitoring of renal function after initiation of concomitant therapy and periodically thereafter.
Diuretics (thiazide or loop diuretics)
Prior treatment with high dose diuretics such as furosemide (loop diuretic) and hydrochlorothiazide (thiazide diuretic) may result in volume depletion, and in a risk of hypotension when initiating therapy with telmisartan.
To be taken into account with concomitant use
Based on their pharmacological properties it can be expected that the following medicinal products may potentiate the hypotensive effects of all antihypertensives including telmisartan: Baclofen, amifostine. Furthermore, orthostatic hypotension may be aggravated by alcohol, barbiturates, narcotics, or antidepressants.
Corticosteroids (systemic use)
Reduction of the antihypertensive effect.
4.6 Use in special populations
Pregnancy
When pregnancy is diagnosed, treatment with BisotrakTM T should be stopped immediately, and, if appropriate, alternative therapy should be started.
Breast-feeding
There are no data on the excretion of BisotrakTM T in human breast milk or the safety of BisotrakTM T exposure in infants. Therefore, breastfeeding is not recommended during administration of BisotrakTM T.
4.7 Effects on ability to drive and use machines
In a study with coronary heart disease patients, BisotrakTM T did not impair driving performance. However, depending on the individual patient’s response to treatment an effect on the ability to drive a vehicle or to use machines cannot be excluded. This needs to be considered particularly at start of treatment, upon change of medication, or in conjunction with alcohol.
4.8 Undesirable effects
Infections and infestations
Uncommon: upper respiratory tract infection including pharyngitis and sinusitis, urinary tract infection including cystitis
Rare: sepsis including fatal outcome
Blood and the lymphatic system disorders
Uncommon: anaemia
Rare: eosinophilia, thrombocytopenia
Immune system disorders
Rare: anaphylactic reaction, hypersensitivity
Metabolism and nutrition disorders
Uncommon: hyperkalaemia
Rare: hypoglycaemia (in diabetic patients)
Psychiatric disorders
Uncommon: depression, insomnia, sleep disorders
Rare: anxiety, nightmares, hallucinations
Nervous system disorders
Common: dizziness, headache
Uncommon: syncope
Rare: somnolence
Eye disorders
Rare: visual disturbance, reduced tear flow (to be considered if the patient uses lenses)
Very rare: conjunctivitis.
Ear and labyrinth disorders
Uncommon: vertigo
Rare: hearing disorders
Cardiac disorders
Very common: bradycardia (in patients with chronic heart failure).
Common: worsening of pre-existing heart failure (in patients with chronic heart failure).
Uncommon: AV-conduction disturbances, worsening of pre-existing heart failure (in patients with hypertension or angina pectoris) ; bradycardia (in patients with hypertension or angina pectoris).
Rare: tachycardia
Vascular disorders
Common: feeling of coldness or numbness in the extremities, hypotension especially in patient with heart failure.
Uncommon: hypotension, orthostatic hypotension
Respiratory, thoracic and mediastinal disorders
Uncommon: dyspnoea, cough, bronchospasm in patients with bronchial asthma or a history of obstructive airways disease.
Rare: allergic rhinitis Very rare: Interstitial lung disease
Gastrointestinal disorders
Common: gastrointestinal complaints such as nausea, vomiting, diarrhoea, constipation.
Uncommon: Abdominal pain, dyspepsia, flatulence,
Rare: Stomach discomfort, dry mouth, dysgeusia
Hepato-biliary disorders
Rare: hepatic function abnormal/liver disorder, hepatitis.
Skin and subcutaneous tissue disorders
Uncommon: hyperhidrosis, pruritus, rash
Rare: Angioedema (also with fatal outcome), eczema, erythema, urticaria, drug eruption, toxic skin eruption
Rare: hypersensitivity reactions (flush and angioedema).
Very rare: beta blockers may provoke or worsen psoriasis or induce psoriasis like rash, alopecia.
Musculoskeletal and connective tissue disorders
Uncommon: myalgia, back pain (e.g. sciatica), muscle spasms, muscular weakness and cramps.
Rare: arthralgia, pain in extremity, tendon pain (tendonitis like symptoms)
Reproductive system and breast disorders
Rare: erectile dysfunction.
Renal and urinary disorders
Common: asthenia (in patients with chronic heart failure), fatigue.
Uncommon: renal impairment including acute renal failure, asthenia (in patients with hypertension or angina pectoris)
General disorders and administration site conditions
Uncommon: chest pain, asthenia (weakness)
Rare: influenza-like illness
Investigations
Uncommon: blood creatinine increased
Rare: blood uric acid increased, hepatic enzyme increased, blood creatine phosphokinase increased, haemoglobin decreased
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com Website: https://www.zuventus.com/drug-safety-reporting By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
The most common signs expected with overdose are bradycardia, tachycardia, hypotension, bronchospasm, acute cardiac insufficiency, hypoglycemia, dizziness, increase in serum creatinine, and acute renal failure. The patient should be closely monitored, and the treatment should be symptomatic and supportive. Management depends on the time since ingestion and the severity of the symptoms. Suggested measures include induction of emesis and / or gastric lavage. Activated charcoal may be useful in the treatment of overdose.
5.0 Pharmacological properties
5.1 Mechanism of action/ Pharmacodynamic properties
Bisoprolol is a potent highly beta1-selective-adrenoceptor blocking agent, lacking intrinsic sympathomimetic and relevant membrane stabilizing activity. It only shows low affinity to the beta2-receptor of the smooth muscles of bronchi and vessels as well as to the beta2-receptors concerned with metabolic regulation. Therefore, bisoprolol is generally not to be expected to influence the airway resistance and beta2-mediated metabolic effects. Its beta1-selectivity extends beyond the therapeutic dose range.
Telmisartan is an orally active and specific angiotensin II receptor (type AT1) antagonist. Telmisartan displaces angiotensin II with very high affinity from its binding site at the AT1 receptor subtype, which is responsible for the known actions of angiotensin II. Telmisartan does not exhibit any partial agonist activity at the AT1 receptor. Telmisartan selectively binds the AT1 receptor. The binding is long-lasting. Telmisartan does not show affinity for other receptors, including AT2 and other less characterized AT receptors. The functional role of these receptors is not known, nor is the effect of their possible overstimulation by angiotensin II, whose levels are increased by telmisartan. Plasma aldosterone levels are decreased by telmisartan. Telmisartan does not inhibit human plasma renin or block ion channels. Telmisartan does not inhibit angiotensin converting enzyme (kininase II), the enzyme which also degrades bradykinin. Therefore, it is not expected to potentiate bradykinin-mediated adverse events.
In human, an 80 mg dose of telmisartan almost completely inhibits the angiotensin II evoked blood pressure increase. The inhibitory effect is maintained over 24 hours and still measurable up to 48 hours.
5.2 Pharmacokinetic Properties
|
Bisoprolol |
Telmisartan |
|
|
Bioavailability |
90% |
50 % |
|
Plasma protein binding |
30 % |
>99.5 % |
|
Volume of distribution (Vdss) |
3.5 l/kg |
500 liters |
|
T1/2 |
10-12 hours |
>20 hours |
|
Elimination |
excreted from the body by two routes. 50% is metabolized by the liver to inactive metabolites which are then excreted by the kidneys. The remaining 50% is excreted by the kidneys in an un metabolized form. |
excreted with the faeces, mainly as unchanged compound. |
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
No known animal toxicology data.
7.0 Description
Bisoprolol Fumarate is a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent. It possesses an asymmetric carbon atom in its structure and is provided as a racemic mixture.
Chemical name: (±)-1-[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]-3-[(1- methylethyl)amino]-2-propanol (E)-2-butenedioate (2:1) (salt)
Molecular formula: (C18H31NO4)2•C4H4O4
Molecular weight: 766.97
Telmisartan Tablets are a non-peptide angiotensin II receptor (type AT1) antagonist.
Chemical name: 4'-[(1,4'-dimethyl-2'-propyl [2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid.
Molecular formula: C33H30N4O2
Molecular weight: 514.63
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf Life
Refer on the pack
8.3 Packaging Information
BisotrakTM T 2.5 mg: Alu-Alu blister strip of 10 tablets.
BisotrakTM T 5 mg: Alu-Alu blister strip of 10 tablets.
8.4 Storage and handing Instructions
Store protected from light & moisture at a temperature not exceeding 30°C. Keep out of reach of children.
9.0 Patient information leaflet
Patients, especially those with coronary artery disease, should be warned about discontinuing use of BisotrakTM T without a physician's supervision. Patients should also be advised to consult a physician if any difficulty in breathing occurs, or if they develop signs or symptoms of congestive heart failure or excessive bradycardia. Patients subject to spontaneous hypoglycemia, or diabetic patients receiving insulin or oral hypoglycemic agents, should be cautioned that beta-blockers may mask some of the manifestations of hypoglycemia, particularly tachycardia, and BisotrakTMT should be used with caution.
Patients should know how they react to this medicine before they operate automobiles and machinery or engage in other tasks requiring alertness.
Pregnancy
Advise female patients of childbearing age about the consequences of exposure to BisotrakTM T during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible.
Lactation
Advise nursing women not to breastfeed during treatment with BisotrakTM T.
Symptomatic Hypotension and Syncope
Advise patients that light headedness can occur, especially during the first days of therapy, and to report it to their healthcare provider. Inform patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of light headedness and possible syncope. Advise patients to contact their healthcare provider if syncope occurs.
12. Date of revision
12.06.2024
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What BisotrakTM T is and what it is used for
- What you need to know before you take BisotrakTM T
- How to take BisotrakTM T
- Possible side effects
- How to store BisotrakTM T
- Contents of the pack and other information
1. What BisotrakTM T is and what it is used for
The active substances in BisotrakTM T is Bisoprolol fumarate and Telmisartan.
Bisoprolol belongs to a group of medicines called beta-blockers. Beta-blocker protects heart from too much activity. This medicine works by affecting the body’s response to some nerve impulses, especially in the heart. As a result, Bisoprolol fumarate slows down the heart rate and makes the heart more efficient at pumping blood around the body.
Telmisartan belongs to a group of medicines called angiotensin II receptor antagonists. Angiotensin-II is a substance produced in your body which causes your blood vessels to narrow thus increasing your blood pressure. Telmisartan blocks the effect of angiotensin II so that the blood vessels relax, and your blood pressure is lowered.
BisotrakTMT is used to treat high blood pressure (hypertension)
2. What you need to know before you take BisotrakTM T
Do not take BisotrakTM T if you:
- are allergic to bisoprolol fumarate or telmisartan
- have severe asthma or severe chronic lung disease
- have a slow or irregular heart rate. Ask your doctor if you are not sure
- have very low blood pressure
- have severe blood circulation problems (such as Raynaud’s syndrome), which may cause your fingers and toes to tingle or turn pale or blue
- have heart failure that suddenly becomes worse and/ or may require hospital treatment
- Cardiogenic shock, which is an acute serious heart condition causing low blood pressure and circulatory failure.
- have excess acid in the blood, a condition known as metabolic acidosis
- have untreated phaeochromocytoma, a rare tumour of the adrenal gland (medulla)
- are more than 3 months pregnant.
- have severe liver problems such as cholestasis or biliary obstruction (problems with the drainage of the bile from the liver and gall bladder) or any other severe liver disease.
- have diabetes or impaired kidney function and you are treated with a blood pressure lowering medicine containing aliskiren
Tell your doctor if you are not sure about any of the above: your doctor will be able to advise you.
Warnings and precautions
Talk to your doctor or pharmacist before taking BisotrakTM T. He or she may want to take special care (for example give additional treatment or perform more frequent checks) if you have any of the following conditions if you:
- have asthma or chronic lung disease
- have diabetes.
- are fasting from solid food
- are treated for hypersensitivity (allergic) reactions.
- have certain heart disease such as disturbance in heart rhythm or severe chest pain at rest (Prinzmetal’s angina)
- have any liver or kidney problems
- have any problems with the circulation in your limbs
- are taking verapamil or diltiazem, medicines used to treat heart conditions. Concomitant use is not recommended
- have (or have had) psoriasis (a recurring skin rash)
- have phaeochromocytoma (tumour of the adrenal gland). Your doctor will need to treat this before prescribing BisotrakTM T for you
- have a thyroid problem. The tablets can hide symptoms of an overactive thyroid
- have renal artery stenosis (narrowing of the blood vessels to one or both kidneys).
- Have heart trouble.
- have raised aldosterone levels (water and salt retention in the body along with imbalance of various blood minerals).
- have Low blood pressure (hypotension), likely to occur if you are dehydrated (excessive loss of body water) or have salt deficiency due to diuretic therapy ('water tablets'), low-salt diet, diarrhoea, or vomiting.
- have elevated potassium levels in your blood.
Consult your doctor if one of the above warnings is applicable to you, or has been in the past. In addition, tell your doctor if you are going to have:
- Desensitization therapy (for example for the prevention of hay fever), because Bisoprolol fumarate may make it more likely that you experience as allergic reaction or such reaction may be more severe.
- Anesthesia (for example for surgery) because this medicine may influence how your body react to this situation.
Children and adolescents
BisotrakTM T is not recommended for use in children or adolescents.
Other medicines and BisotrakTM T
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Certain medicines cannot be used at the same time, while other drugs require specific changes (to the dose, for example).
Do not take the following medicines with BisotrakTM T without special advice from your doctor:
- Medicines for controlling the blood pressure or medicines for heart problems (such as amiodarone, amlodipine, clonidine, digitalis glycosides, diltiazem, disopyramide, felodipine, flecainide, lidocaine, methyldopa, moxonidine, phenytoin, propafenone, quinidine, rilmenidine, verapamil)
- Medicines for depression e.g. imipramine, amitriptyline, moclobemide
- Medicines to treat mental illness e.g. phenothiazines such as levomepromazine
- Medicines used for anaesthesia during an operation (see also “Warnings and precautions”)
- Medicines used to treat epilepsy e.g. barbiturates such as phenobarbital
- Certain pain killers (for instance acetyl salicylic acid, diclofenac, indomethacin, ibuprofen, naproxen)
- Medicines for asthma or medicines used for a blocked nose
- Medicines used for certain eye disorders such as glaucoma (increased pressure in the eye) or used to widen the pupil of the eye.
- Certain medicines to treat clinical shock (e. g. adrenaline, dobutamine, noradrenaline)
- Mefloquine, a medicine for malaria
- Lithium containing medicines to treat some types of depression.
- Medicines that may increase blood potassium levels such as salt substitutes containing potassium potassium-sparing diuretics (certain 'water tablets'), ACE inhibitors, angiotensin II receptor antagonists, NSAIDs (non-steroidal anti-inflammatory medicines, e.g. aspirin or ibuprofen), heparin, immunosuppressives (e.g. cyclosporin or tacrolimus), and the antibiotic trimethoprim.
- Diuretics ('water tablets'), especially if taken in high doses together with BisotrakTM T, may lead to excessive loss of body water and low blood pressure (hypotension).
- If you are taking an ACE-inhibitor or aliskiren.
- Digoxin
- NSAIDs (non-steroidal anti-inflammatory medicines, e.g. aspirin or ibuprofen) or corticosteroids
All these drugs as well as bisoprolol may influence the blood pressure and/or heart function.
- Rifampicin for the treatment of infections
- medicines to treat severe headaches or migraines (ergotamine derivatives).
It is also especially important to speak with your doctor if you are taking:
- Insulin or other products for diabetes. The blood glucose reducing effect may be enhanced. Symptoms of low blood glucose level can be masked.
- other medicines used to treat high blood pressure or of medicines with blood pressure lowering potential (e.g. baclofen, amifostine).
- alcohol, barbiturates, narcotics or antidepressants. (You may notice this as dizziness when standing up. You should consult with your doctor if you need to adjust the dose of your other medicine while taking BisotrakTM T.
Bisoprolol Fumarate with food, drink and alcohol
BisotrakTM T may be taken with or without food and should be swallowed whole with a drink of water.
Pregnancy, breast-feeding and fertility
Do not take BisotrakTM T, if you are pregnant or may be pregnant. BisotrakTM T may be harmful to the pregnancy and/or the unborn child. There is an increased possibility of premature birth, miscarriage, low blood sugar level and reduced heart rate of the child. The growth of the baby may also be affected.
BisotrakTM T is not recommended for mothers who are breast-feeding, and your doctor may choose another treatment for you if you wish to breast-feed, especially if your baby is newborn, or was born prematurely.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
These tablets may make you feel tired, drowsy or dizzy. If you suffer from these side effects, do not operate vehicles and/or machines. Be aware of the possibility of these effects, particularly at the beginning of the treatment, with changes in medication and with use in combination with alcohol.
3. How to take BisotrakTM T
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose of BisotrakTM T is one tablet a day.
Your doctor will adjust BisotrakTM T Tablets dosage according to your need, preferably starting with lower dose and titrate to higher dose in cases where the target blood pressure is not achieved, the dose of Telmisartan can be increased to a maximum of 80 mg and accordingly Bisoprolol Fumarate10 mg once daily.
If you take more BisotrakTM T than you should
If you have accidentally taken more than the prescribed dose, tell your doctor/pharmacist immediately. Take any remaining tablets or this leaflet with you so the medical staff know exactly what you have taken. Symptoms of overdose may include dizziness, light-headedness, fatigue, breathlessness and/or wheezing. Also, there may be reduced heart rate, reduced blood pressure, insufficient action of the heart and a low blood glucose level (which may involve feelings of hunger, sweating and palpitations).
If you forget to take BisotrakTM T
Do not take a double dose to make up for a forgotten dose. Take the normal dose as soon as you remember and then carry on with the usual normal dose the next day. If you stop taking BisotrakTM T Treatment with BisotrakTM T must not be stopped abruptly. If you suddenly stop taking this medicine your condition may get worse. The dose of BisotrakTM T must be reduced gradually over a few weeks as advised by your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
To prevent serious reaction, speak to a doctor immediately if a side effect is severe, occurs suddenly or gets worse rapidly. The most serious side effects are related to the heart function:
- Slowing of heart rate (affect more than 1 patient in 10 in patient with chronic heart failure and affected less than 1 patient in 100 in patient with hypertension or angina pectoris)
- Worsening of heart failure (affects more than 1 patient in 100 in patient with chronic heart failure and affected less than 1 patient in 100 in patient with hypertension or angina pectoris)
- irregular heartbeat (affects less than 1 patient in 100)
- Worsening of symptom of blockage of the main blood vessel to the legs, especially at the start of treatment (Frequency not stated).
- Sepsis# (often called "blood poisoning", is a severe infection with whole-body inflammatory response), rapid swelling of the skin and mucosa (angioedema); these side effects are rare but are extremely serious and patients should stop taking the product and see their doctor immediately. If these effects are not treated they could be fatal.
If you feel dizzy or weak or have breathing difficulties, please contact your doctor as soon as possible.
Further side effects are listed below according to how frequently they may occur:
Common (may affect up to 1 in 10 people):
- Tiredness*, feeling weak, (In patient with chronic heart failure), dizziness*, headache* • Feeling of coldness or numbness in hands or feet
- low blood pressure, especially in patient with heart failure.
- Stomach or intestine problem such as nausea, vomiting, diarrhoea or constipation
Uncommon (may affect up to 1 in 100 people):
- Sleep disturbances
- Depression
- Breathing problems in patients with asthma or chronic lung disease
- Muscle weakness, muscle cramps
- feeling weak (In patient with hypertension or angina pectoris)
- Urinary tract infections, upper respiratory tract infections (e.g. sore throat, inflamed sinuses, common cold), deficiency in red blood cells (anaemia), high potassium levels, difficulty falling asleep, feeling sad (depression), fainting (syncope), feeling of spinning (vertigo), slow heart rate (bradycardia), low blood pressure (hypotension), in users treated for high blood pressure, dizziness on standing up (orthostatic hypotension), shortness of breath, cough, abdominal pain, diarrhoea, discomfort in the abdomen, bloating, vomiting, itching, increased sweating, drug rash, back pain, muscle cramps, muscle pain (myalgia), kidney impairment including acute kidney failure, pain in the chest, feeling of weakness, and increased level of creatinine in the blood.
Rare: (may affect up to 1 in 1,000 people):
- Hearing problems
- Inflammation of the lining of the nose, causing a runny nose with irritation
- Allergic reactions (itching, flushed appearance). You should see your doctor straight away if you experience more severe allergic reactions, which may involve face, neck, tongue, mouth or throat swelling, or difficulty breathing.
- Dry eyes from reduced tear flow (can be very troublesome if you use contact lenses)
- Inflammation of the liver (hepatitis), causing abdominal pain, loss of appetite and sometimes jaundice with yellowing of the whites of the eyes and skin and dark urine
- Reduced sexual performance (impaired erection)
- certain blood test results for liver function or fat levels differing from normal
- fainting
- Increase in certain white blood cells (eosinophilia), low platelet count (thrombocytopenia), severe allergic reaction (anaphylactic reaction), allergic reaction (e.g. rash, itching, difficulty breathing, wheezing, swelling of the face or low blood pressure), low blood sugar levels (in diabetic patients),
- feeling anxious, somnolence, impaired vision, fast heart beat (tachycardia), upset stomach, dry mouth, taste disturbance (dysgeusia), abnormal liver function (Japanese patients are more likely to experience these side effect), rapid swelling of the skin and mucosa which can also lead to death (angioedema also with fatal outcome),
- eczema (a skin disorder), redness of skin, hives (urticaria), severe drug rash, joint pain (arthralgia), pain in extremity, tendon pain, flu-like-illness, decreased haemoglobin (a blood protein), increased levels of uric acid, increased hepatic enzymes or creatine phosphokinase in the blood.
Very Rare: (may affect up to 1 in 10,000 people):
- Irritation and redness of eye (conjunctivitis)
- Hair loss
- Appearance or worsening of scaly skin rash (psoriasis): Psoriasis like rash.
- Progressive scarring of lung tissue (interstitial lung disease) **
# The event may have happened by chance or could be related to a mechanism currently not known.
**Cases of progressive scarring of lung tissue have been reported during intake of telmisartan.
However, it is not known whether telmisartan was the cause.
* if treated for high blood pressure or angina then these symptoms occur especially at beginning of treatment, or if your dosage changes. They are generally mild or often disappear within 1 to 2 weeks.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store BisotrakTM T
Keep out of reach of children.
Store below 30 ºC. Store in the original package in order to protect from light.
Do not use BisotrakTM T after the expiry date which is stated on the strips after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What BisotrakTM T contains
The active substance is bisoprolol fumarate and Telmisartan.
Each film coated tablet contains:
Bisoprolol Fumarate IP 2.5/5 mg
Telmisaratan IP 40 mg
Packaging:
BisotrakTM T 2.5 mg: Alu-Alu blister strip of 10 tablets.
BisotrakTM T 5 mg: Alu-Alu blister strip of 10 tablets.
Marketing Authorisation Holder
Zuventus Healthcare Limited Zuventus House, Plot Y2, CTS No.: 358/A2, Near Nahur Railway Station, Nahur (W), Mumbai, 400078 Maharashtra, India
This leaflet was last revised in 06/2024
© Zuventus Healthcare Ltd., 2020. All rights reserved.