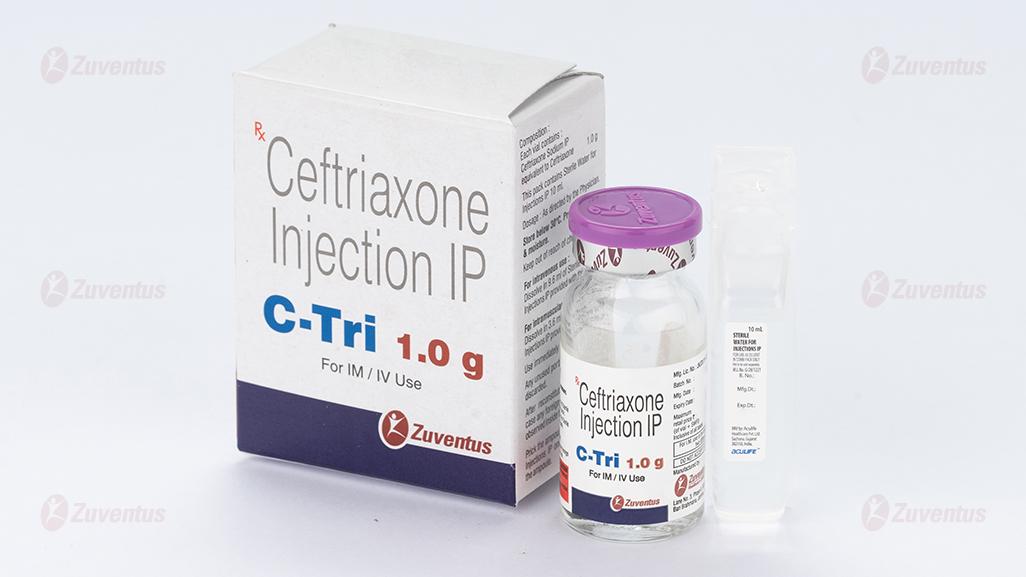
C Tri 1.0g Injection
Therapy Area
Anti Bacterial
1.0 Generic name
Ceftriaxone Injection IP
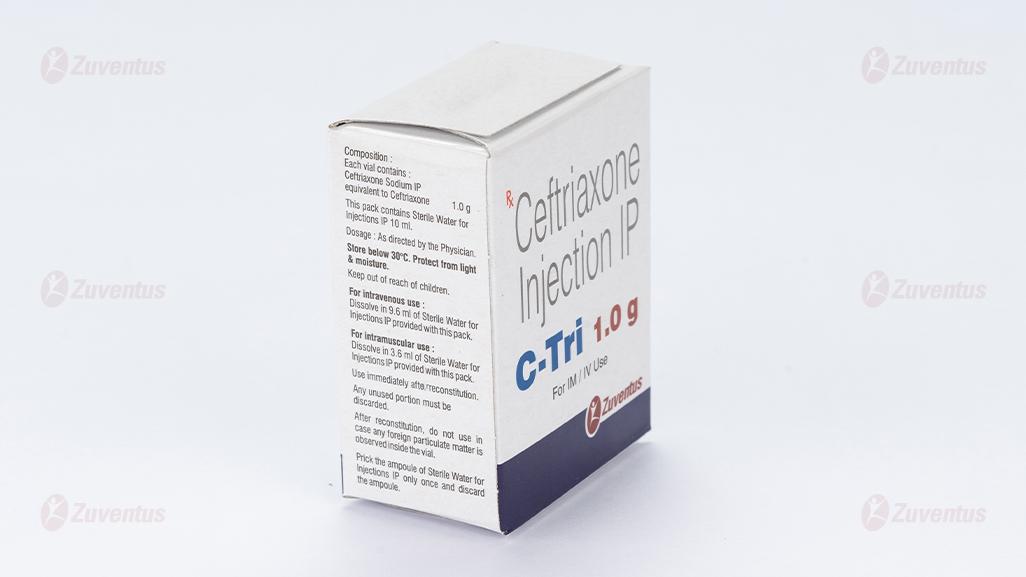
2.0 Qualitative and quantitative composition
C Tri 125 / 250 / 500 / 1.0 g / 2.0 g
Each vial contains :
Ceftriaxone Sodium IP
equivalent to Ceftriaxone 125 mg / 250 mg / 500 mg / 1.0 g / 2.0 g
This pack contains Sterile Water for Injections IP 5/5/5/10/10 ml.
3.0 Dosage form and strength
Injection, [125 / 250 / 500 / 1.0 g / 2.0 g Injection]
4.0 Clinical particulars
4.1 Therapeutic indication
- Urinary tract infection (UTI)
- Lower respiratory tract infections
- Bacteraemia
- Septicaemia
- Meningitis
- Abdominal infections and infections caused by pseudomonas species.
- Skin and skin structure infections
- Acute Bacterial Otitis Media
- Surgical Prophylaxis
- Uncomplicated Gonorrhea
4.2 Posology and method of administration
Ceftriaxone for injection may be administered intravenously or intramuscularly. Do not use diluents containing calcium, such as Ringer's solution or Hartmann's solution, to reconstitute ceftriaxone for injection vials or to further dilute a reconstituted vial for IV administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone for injection is mixed with calcium-containing solutions in the same IV administration line. Ceftriaxone for injection must not be administered simultaneously with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone for injection and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid.
There have been no reports of an interaction between ceftriaxone and oral calcium-containing products or interaction between intramuscular ceftriaxone and calcium-containing products (IV or oral).
Neonates
Hyperbilirubinemic neonates, especially prematures, should not be treated with ceftriaxone for injection. Ceftriaxone for injection is contraindicated in premature neonates. Ceftriaxone for injection is contraindicated in neonates (≤ 28 days) if they require (or are expected to require) treatment with calcium-containing IV solutions, including continuous calcium- containing infusions such as parenteral nutrition because of the risk of precipitation of ceftriaxone-calcium. Intravenous doses should be given over 60 minutes in neonates to reduce the risk of bilirubin encephalopathy.
Pediatric Patients
For the treatment of skin and skin structure infections, the recommended total daily dose is 50 to 75 mg/kg given once a day (or in equally divided doses twice a day). The total daily dose should not exceed 2 grams. For the treatment of acute bacterial otitis media, a single intramuscular dose of 50 mg/kg (not to exceed 1 gram) is recommended. For the treatment of serious miscellaneous infections other than meningitis, the recommended total daily dose is 50 to 75 mg/kg, given in divided doses every 12 hours. The total daily dose should not exceed 2 grams. In the treatment of meningitis, it is recommended that the initial therapeutic dose be 100 mg/kg (not to exceed 4 grams). Thereafter, a total daily dose of 100 mg/kg/day (not to exceed 4 grams daily) is recommended. The daily dose may be administered once a day (or in equally divided doses every 12 hours). The usual duration of therapy is 7 to 14 days.
Adults
The usual adult daily dose is 1 to 2 grams given once a day (or in equally divided doses twice a day) depending on the type and severity of infection. The total daily dose should not exceed 4 grams. If Chlamydia trachomatis is a suspected pathogen, appropriate antichlamydial coverage should be added, because ceftriaxone sodium has no activity against this organism. For the treatment of uncomplicated gonococcal infections, a single intramuscular dose of 250 mg is recommended. For preoperative use (surgical prophylaxis), a single dose of 1 gram administered intravenously 1/2 to 2 hours before surgery is recommended. Generally, ceftriaxone for injection therapy should be continued for at least 2 days after the signs and symptoms of infection have disappeared. The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required. When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days. No dosage adjustment is necessary for patients with impairment of renal or hepatic function. The dosages recommended for adults require no modification in elderly patients, up to 2 g per day, provided there is no severe renal and hepatic impairment.
Directions for Use
C Tri 125
For intravenous use Dissolve in 2.0 ml of Sterile Water for Injections IP provided with this pack. Any unused portion must be discarded. For intramuscular use Dissolve in 0.5 ml of Sterile Water for Injections IP provided with this pack
C Tri 250
For intravenous use Dissolve in 2.4 ml of Sterile Water for Injections IP provided with this pack. Any unused portion must be discarded. For intramuscular use Dissolve in 0.9 ml of Sterile Water for Injections IP provided with this pack
C Tri 500
For intravenous use Dissolve in 4.8 ml of Sterile Water for Injections IP provided with this pack. Any unused portion must be discarded. For intramuscular use Dissolve in 1.8 ml of Sterile Water for Injections IP provided with this pack.
C Tri 1.0 g
For intravenous use Dissolve in 9.6 ml of Sterile Water for Injections IP provided with this pack. Any unused portion must be discarded. For intramuscular use Dissolve in 3.6 ml of Sterile Water for Injections IP provided with this pack
C Tri 2.0 g
For intravenous use Dissolve in 10.0 ml of Sterile Water for Injections IP provided with this pack. After reconstitution each ml of solution contains approximately 200 mg of Ceftriaxone. Withdraw entire contents and dilute with I.V. diluents. For intramuscular use Dissolve in 7.2 ml of Sterile Water for Injections IP provided with this pack
4.3 Contraindications
Hypersensitivity
- Ceftriaxone for injection is contraindicated in patients with known hypersensitivity to ceftriaxone, any of its excipients or to any other cephalosporin.
- Patients with previous hypersensitivity reactions to penicillin and other beta lactam antibacterial agents may be at greater risk of hypersensitivity to ceftriaxone.
Neonates
Premature neonates
Ceftriaxone for injection is contraindicated in premature neonates up to a postmenstrual age of 41 weeks (gestational age + chronological age).
Hyperbilirubinemic neonates
Hyperbilirubinemic neonates should not be treated with ceftriaxone for injection. Ceftriaxone can displace bilirubin from its binding to serum albumin, leading to a risk of bilirubin encephalopathy in these patients.
Neonates requiring calcium containing IV solutions
Ceftriaxone for injection is contraindicated in neonates (≤ 28 days) if they require (or are expected to require) treatment with calcium-containing IV solutions, including continuous calcium- containing infusions such as parenteral nutrition because of the risk of precipitation of ceftriaxone-calcium. Cases of fatal outcomes in which a crystalline material was observed in the lungs and kidneys at autopsy have been reported in neonates receiving ceftriaxone for injection and calcium-containing fluids. In some of these cases, the same intravenous infusion line was used for both ceftriaxone for injection and calcium-containing fluids and in some a precipitate was observed in the intravenous infusion line. There have been no similar reports in patients other than neonates.
4.4 Special warnings and precautions for use
Hypersensitivity reactions
Before therapy with ceftriaxone for injection is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cephalosporins, penicillins and other beta-lactam agents or other drugs. This product should be given cautiously to penicillin and other beta-lactam agent-sensitive patients. Antibacterial drugs should be administered with caution to any patient who has demonstrated some form of allergy, particularly to drugs. Serious acute hypersensitivity reactions may require the use of subcutaneous epinephrine and other emergency measures.
As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions (i.e., anaphylaxis) have been reported. In case of severe hypersensitivity reactions, treatment with ceftriaxone must be discontinued immediately and adequate emergency measures must be initiated.
Interaction with calcium-containing products
Do not use diluents containing calcium, such as Ringer's solution or Hartmann's solution, to reconstitute ceftriaxone for injection vials or to further dilute a reconstituted vial for IV administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone for injection is mixed with calcium-containing solutions in the same IV administration line. Ceftriaxone for Injection must not be administered simultaneously with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone for injection and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid. In vitro studies using adult and neonatal plasma from umbilical cord blood demonstrated that neonates have an increased risk of precipitation of ceftriaxone-calcium.
Neurological adverse reactions
Serious neurological adverse reactions have been reported during postmarketing surveillance with ceftriaxone use. These reactions include encephalopathy (disturbance of consciousness including somnolence, lethargy, and confusion), seizures, myoclonus, and non-convulsive status epilepticus. Some cases occurred in patients with severe renal impairment who did not receive appropriate dosage adjustment. However, in other cases, neurological adverse reactions occurred in patients receiving an appropriate dosage adjustment. The neurological adverse reactions were reversible and resolved after discontinuation. If neurological adverse reactions associated with Ceftriaxone for Injection therapy occur, discontinue Ceftriaxone for Injection and institute appropriate supportive measures. Make appropriate dosage adjustments in patients with severe renal impairment.
Clostridium difficile - associated diarrhea Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ceftriaxone for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Hemolytic anemia
An immune mediated hemolytic anemia has been observed in patients receiving cephalosporin class antibacterials including ceftriaxone for injection. Severe cases of hemolytic anemia, including fatalities, have been reported during treatment in both adults and children. If a patient develops anemia while on ceftriaxone, the diagnosis of a cephalosporin associated anemia should be considered and ceftriaxone stopped until the etiology is determined.
Development of drug-resistant bacteria
Prescribing ceftriaxone for injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. Prolonged use of ceftriaxone for injection may result in overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Patients with renal or hepatic impairment
Ceftriaxone is excreted via both biliary and renal excretion. Therefore, patients with renal failure normally require no adjustment in dosage when usual doses of ceftriaxone for injection are administered. Dosage adjustments should not be necessary in patients with hepatic dysfunction; however, in patients with both hepatic dysfunction and significant renal disease, caution should be exercised and the ceftriaxone for injection dosage should not exceed 2 g daily. Ceftriaxone is not removed by peritoneal- or hemodialysis. In patients undergoing dialysis no additional supplementary dosing is required following the dialysis. In patients with both severe renal and hepatic dysfunction, close clinical monitoring for safety and efficacy is advised.
Effect on prothrombin time
Alterations in prothrombin times have occurred in patients treated with ceftriaxone for injection. Monitor prothrombin time during ceftriaxone for injection treatment in patients with impaired vitamin K synthesis or low vitamin K stores (eg, chronic hepatic disease and malnutrition). Vitamin K administration (10 mg weekly) may be necessary if the prothrombin time is prolonged before or during therapy. Concomitant use of ceftriaxone with Vitamin K antagonists may increase the risk of bleeding. Coagulation parameters should be monitored frequently, and the dose of the anticoagulant adjusted accordingly, both during and after treatment with ceftriaxone .
Gallbladder pseudolithiasis
Ceftriaxone-calcium precipitates in the gallbladder have been observed in patients receiving ceftriaxone for injection. These precipitates appear on sonography as an echo without acoustical shadowing suggesting sludge or as an echo with acoustical shadowing which may be misinterpreted as gallstones. The probability of such precipitates appears to be greatest in pediatric patients. Patients may be asymptomatic or may develop symptoms of gallbladder disease. The condition appears to be reversible upon discontinuation of ceftriaxone sodium and institution of conservative management. Discontinue ceftriaxone sodium in patients who develop signs and symptoms suggestive of gallbladder disease and/or the sonographic findings described above.
Urolithiasis and post-renal acute renal failure
Ceftriaxone-calcium precipitates in the urinary tract have been observed in patients receiving ceftriaxone for injection and may be detected as sonographic abnormalities. The probability of such precipitates appears to be greatest in pediatric patients. Patients may be asymptomatic or may develop symptoms of urolithiasis, and ureteral obstruction and post-renal acute renal failure. The condition appears to be reversible upon discontinuation of ceftriaxone sodium and institution of appropriate management. Ensure adequate hydration in patients receiving ceftriaxone for injection. Discontinue ceftriaxone for injection in patients who develop signs and symptoms suggestive of urolithiasis, oliguria or renal failure and/or the sonographic findings described above.
Pancreatitis
Cases of pancreatitis, possibly secondary to biliary obstruction, have been reported in patients treated with ceftriaxone for injection. Most patients presented with risk factors for biliary stasis and biliary sludge (preceding major therapy, severe illness, total parenteral nutrition). A cofactor role of ceftriaxone for injection-related biliary precipitation cannot be ruled out.
4.5 Drugs interactions
Calcium-containing diluents, such as Ringer's solution or Hartmann's solution, should not be used to reconstitute Ceftriaxone vials or to further dilute a reconstituted vial for intravenous administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone is mixed with calciumcontaining solutions in the same intravenous administration line. Ceftriaxone must not be administered simultaneously with calcium-containing intravenous solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between
infusions with a compatible fluid. In vitro studies using adult and neonatal plasma from umbilical cord blood demonstrated that neonates have an increased risk of precipitation of ceftriaxone-calcium. Concomitant use with oral anticoagulants may increase the anti-vitamin K effect and the risk of bleeding. It is recommended that the International Normalised Ratio (INR) is monitored frequently and the posology of the anti-vitamin K drug adjusted accordingly, both during and after treatment with ceftriaxone.
There is conflicting evidence regarding a potential increase in renal toxicity of aminoglycosides when used with cephalosporins. The recommended monitoring of aminoglycoside levels (and renal function) in clinical practice should be closely adhered to in such cases. In an in-vitro study antagonistic effects have been observed with the combination of chloramphenicol and ceftriaxone. The clinical relevance of this finding is unknown. There have been no reports of an interaction between ceftriaxone and oral calcium-containing products or interaction between intramuscular ceftriaxone and calcium-containing products (intravenous or oral).
In patients treated with ceftriaxone, the Coombs' test may lead to false-positive test results. Ceftriaxone, like other antibiotics, may result in false-positive tests for galactosaemia. Likewise, non-enzymatic methods for glucose determination in urine may yield false-positive results. For this reason, glucose level determination in urine during therapy with ceftriaxone should be carried out enzymatically. No impairment of renal function has been observed after concurrent administration of large doses of ceftriaxone and potent diuretics (e.g. furosemide). Simultaneous administration of probenecid does not reduce the elimination of ceftriaxone.
4.6 Use in special populations
Pregnancy
Reproductive studies have been performed in mice and rats at doses up to 20 times the usual human dose and have no evidence of embryotoxicity, fetotoxicity or teratogenicity. In primates, no embryotoxicity or teratogenicity was demonstrated at a dose approximately 3 times the human dose. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing mothers
Low concentrations of ceftriaxone are excreted in human milk. Caution should be exercised when ceftriaxone for injection is administered to a nursing woman.
Pediatric use
In vitro studies have shown that ceftriaxone, like some other cephalosporins, can displace bilirubin from serum albumin. Ceftriaxone for injection should not be administered to hyperbilirubinemic neonates, especially prematures.
4.7 Effects on ability to drive and use machines
During treatment with ceftriaxone, undesirable effects may occur (e.g. dizziness), which may influence the ability to drive and use machines. Patients should be cautious when driving or operating machinery
4.8 Undesirable effects
Ceftriaxone for injection is generally well tolerated. In clinical trials, the following adverse reactions, which were considered to be related to ceftriaxone for injection therapy or of uncertain etiology, were observed :
Local reactions : Pain, induration, tenderness, phlebitis; General disorders and administration site conditions : Injection site pain; Hypersensitivity : Rash. Less frequently reported were pruritus, fever or chills; Infections and Infestations : Genital fungal infection Hematologic : Eosinophilia, thrombocytosis and leukopenia. Less frequently reported were anemia, hemolytic anemia, neutropenia, lymphopenia, thrombocytopenia and prolongation of the prothrombin time; Blood and Lymphatic disorders : Granulocytopenia, coagulopathy; Gastrointestinal : Diarrhea/loose stools. Less frequently reported were nausea or vomiting, and dysgeusia. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment; Hepatic : Elevations of aspartate aminotransferase (AST) or alanine aminotransferase (ALT). Less frequently reported were elevations of alkaline phosphatase and bilirubin; Renal : Elevations of the BUN. Less frequently reported were elevations of creatinine and the presence of casts in the urine. Central Nervous System : Headache or dizziness were reported occasionally; Genitourinary : Moniliasis or vaginitis were reported occasionally. Skin and mucous membranes : Stevens-Johnson Syndrome (SJS), Fixed Drug Eruption (FDE). Miscellaneous : Diaphoresis and flushing were reported occasionally. Other rarely observed adverse reactions include abdominal pain, agranulocytosis, allergic pneumonitis, anaphylaxis, basophilia, biliary lithiasis, bronchospasm, colitis, dyspepsia, epistaxis, flatulence, gallbladder sludge, glycosuria, hematuria, jaundice, leukocytosis, lymphocytosis, monocytosis, nephrolithiasis, palpitations, a decrease in the prothrombin time, renal precipitations, seizures, and serum sickness. Investigations : Blood creatinine increased. Cephalosporin class adverse reactions In addition to the adverse reactions listed above which have been observed in patients treated with ceftriaxone, the following adverse reactions and altered laboratory test results have been reported for cephalosporin class antibiotics :
Adverse reactions
Allergic reactions, drug fever, serum sickness-like reaction, renal dysfunction, toxic nephropathy, reversible hyperactivity, hypertonia, hepatic dysfunction including cholestasis, aplastic anemia, hemorrhage, and superinfection.
Altered laboratory tests
Positive direct Coombs' test, false-positive test for urinary glucose, and elevated LDH. Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Reporting of suspected adverse reactions
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly : Website : www.zuventus.com and click the tab “Safety Reporting” located on the top of the home page. By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
4.9 Overdose
Ceftriaxone overdosage has been reported in patients with severe renal impairment. Reactions have included neurological outcomes, including encephalopathy, seizures, myoclonus, and non convulsive status epilepticus. In the event of overdosage, discontinue Ceftriaxone for Injection therapy and provide general supportive treatment. In the case of overdosage, drug concentration would not be reduced by hemodialysis or peritoneal dialysis. There is no specific antidote. Treatment of overdosage should be symptomatic.
5.0 Pharmacological properties
5.1 Mechanism of action
Ceftriaxone inhibits bacterial cell wall synthesis following attachment to penicillin binding proteins (PBPs). This results in the interruption of cell wall (peptidoglycan) biosynthesis, which leads to bacterial cell lysis and death.
5.2 Pharmacodynamic properties
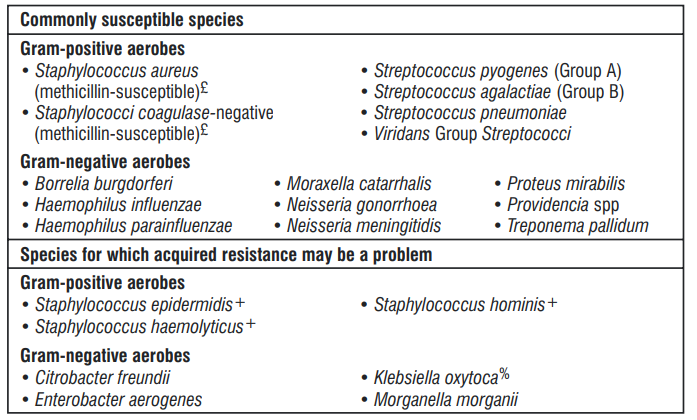
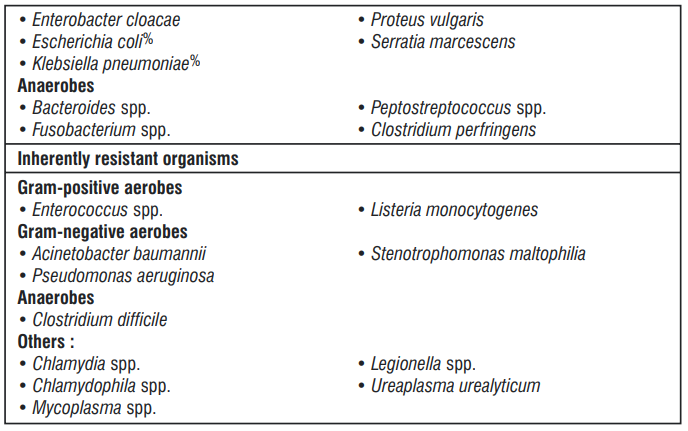
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of ceftriaxone in at least some types of infections is questionable.

5.3 Pharmacokinetic properties
Absorption
Intramuscular administration
Following intramuscular injection, mean peak plasma ceftriaxone levels are approximately half those observed after intravenous administration of an equivalent dose. The maximum plasma concentration after a single intramuscular dose of 1 g is about 81 mg/l and is reached in 2 - 3 hours after administration.
The area under the plasma concentration-time curve after intramuscular administration is equivalent to that after intravenous administration of an equivalent dose. Intravenous administration
After intravenous bolus administration of ceftriaxone 500 mg and 1 g, mean peak plasma ceftriaxone levels are approximately 120 and 200 mg/l respectively. After intravenous infusion of ceftriaxone 500 mg, 1 g and 2 g, the plasma ceftriaxone levels are approximately 80, 150 and 250 mg/l respectively.
Distribution
The volume of distribution of ceftriaxone is 7 - 12 l. Concentrations well above the minimal inhibitory concentrations of most relevant pathogens are detectable in tissue including lung, heart, biliary tract/liver, tonsil, middle ear and nasal mucosa, bone, and in cerebrospinal, pleural, prostatic and synovial fluids. An 8 - 15 % increase in mean peak plasma concentration (Cmax) is seen on repeated administration; steady state is reached in most cases within 48 - 72 hours depending on the route of administration.
Penetration into particular tissues
Ceftriaxone penetrates the meninges. Penetration is greatest when the meninges are inflamed. Mean peak ceftriaxone concentrations in CSF in patients with bacterial meningitis are reported to be up to 25 % of plasma levels compared to 2 % of plasma levels in patients with uninflamed meninges. Peak ceftriaxone concentrations in CSF are reached approximately 4-6 hours after intravenous injection. Ceftriaxone crosses the placental barrier and is excreted in the breast milk at low concentrations.
Protein binding
Ceftriaxone is reversibly bound to albumin. Plasma protein binding is about 95 % at plasma concentrations below 100 mg/l. Binding is saturable and the bound por tion decreases with rising concentration (up to 85 % at a plasma concentration of 300 mg/l).
Biotransformation
Ceftriaxone is not metabolised systemically; but is converted to inactive metabolites by the gut flora.
Elimination
Plasma clearance of total ceftriaxone (bound and unbound) is 10 - 22 ml/min. Renal clearance is 5 - 12 ml/min. 50 - 60 % of ceftriaxone is excreted unchanged in the urine, primarily by glomerular filtration, while 40 - 50 % is excreted unchanged in the bile. The elimination half-life of total ceftriaxone in adults is about 8 hours.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
There is evidence from animal studies that high doses of ceftriaxone calcium salt led to formation of concrements and precipitates in the gallbladder of dogs and monkeys, which proved to be reversible. Animal studies produced no evidence of toxicity to reproduction and genotoxicity. Carcinogenicity studies on ceftriaxone were not conducted.
7.0 Description
Ceftriaxone for injection is a sterile, semisynthetic, broad-spectrum cephalosporin antibiotic for intravenous or intramuscular administration. Ceftriaxone sodium is (6R,7R)-7-[2-(2-Amino-4- thiazolyl)glyoxylamido]-8-oxo-3-[[(1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-as-triazin-3- yl)thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 72-(Z)-(O-methyloxime), disodium salt, sesquaterhydrate.
The chemical formula of ceftriaxone sodium is C18H16N8Na2O7S3·3.5H2O. It has a calculated molecular weight of 661.59 g/mol
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
C Tri 125 : A vial of 125 mg with SWFI IP 5 ml.
C Tri 250 : A vial of 250 mg with SWFI IP 5 ml.
C Tri 500 : A vial of 500 mg with SWFI IP 5 ml
C Tri 1.0 g : A vial of 1.0 g with SWFI IP 10 ml
C Tri 2.0 g : A vial of 2.0 g with SWFI IP 10 ml.
8.4 Storage and handing instructions
Store below 30°C. Protect from light & moisture.
Keep out of reach of children
9.0 Patient counselling information
- Advise patients that neurological adverse reactions could occur with Ceftriaxone for Injection use. Instruct patients or their caregivers to inform their healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including somnolence, lethargy, and confusion), seizures, myoclonus, and nonconvulsive status epilepticus, for immediate treatment, or discontinuation of Ceftriaxone for Injection.
- Patients should be counseled that antibacterial drugs including ceftriaxone for injection should only be used to treat bacterial infections. They do not treat viral infections (eg, common cold).
- When ceftriaxone for injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ceftriaxone for injection or other antibacterial drugs in the future.
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop water y and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
12.0 Date of revision
03 February 2023
About leaflet
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only.
- Do not pass it on to others.
- It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What C-Tri is and what it is used for
- What you need to know before you are given C-Tri
- How C-Tri is given
- Possible side effects
- How to store C-Tri
- Contents of the pack and other information
1. What C-Tri is and what it is used for
C-Tri is an antibiotic given to adults and children (including newborn babies). It works by killing bacteria that cause infections. It belongs to a group of medicines called cephalosporins.
C-Tri is used to treat infections of
- The brain (meningitis).
- the lungs. the middle ear.
- the abdomen and abdominal wall (peritonitis).
- the urinary tract and kidneys.
- bones and joints. the skin or soft tissues.
- the blood.
- the heart.
It can be given:
- to treat specific sexually transmitted infections (gonorrhoea and syphilis).
- to treat patients with low white blood cell counts (neutropenia) who have fever due to bacterial infection.
- to treat infections of the chest in adults with chronic bronchitis.
- to treat Lyme disease (caused by tick bites) in adults and children including newborn babies from 15 days of age.
- to prevent infections during surgery.
2. What you need to know before you are given C-Tri
You must not be given C-Tri if:
- You are allergic to ceftriaxone or any of the other ingredients of this medicine (listed in section 6).
- You have had a sudden or severe allergic reaction to penicillin or similar antibiotics (such as cephalosporins, carbapenems or monobactams). The signs include sudden swelling of the throat or face which might make it difficult to breath or swallow, sudden swelling of the hands, feet and ankles, and a severe rash that develops quickly.
- You are allergic to lidocaine and you are to be given C-Tri as an injection into a muscle.
C-Tri must not be given to babies if:
- The baby is premature.
- The baby is newborn (up to 28 days of age) and has certain blood problems or jaundice (yellowing of the skin or the whites of the eyes) or is to be given a product that contains calcium into their vein.
Warnings and precautions
- Talk to your doctor or pharmacist or nurse before you are given C-Tri if:
- You have recently received or are about to receive products that contain calcium.
- You have recently had diarrhoea after having an antibiotic medicine. You have ever had problems
- with your gut, in particular colitis (inflammation of the bowel).
- You have liver or kidney problems (see section 4).
- You have gall stones or kidney stones
- You have other illnesses, such as haemolytic anaemia (a reduction in your red blood cells that
- may make your skin pale yellow and cause weakness or breathlessness).
- You are on a low sodium diet.
- You experience or have previously experienced a combination of any of the following symptoms:
- rash, red skin, blistering of the lips eyes and mouth, skin peeling, high fever, flu-like
- symptoms, increased levels of liver enzymes seen in blood tests and an increase in a type of
- white blood cell (eosinophilia) and enlarged lymph nodes (signs of severe skin reactions, see
- also section 4 “Possible side effects”).
If you need a blood or urine test
If you are given C-Tri for a long time, you may need to have regular blood tests. C-Tri can affect the results of urine tests for sugar and a blood test known as the Coombs test. If you are having tests:
- Tell the person taking the sample that you have been given C-Tri.
If you are diabetic or need to have your blood glucose level monitored, you should not use certain blood glucose monitoring systems which may estimate blood glucose incorrectly while you are receiving ceftriaxone. If you use such systems check the instructions for use and tell your doctor, pharmacist or nurse. Alternative testing methods should be used if necessary.
Children
Talk to your doctor or pharmacist or nurse before your child is administered C-Tri if:
He/She has recently been given or is to be given a product that contains calcium into their vein.
Other medicines and C-Tri
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
- A type of antibiotic called an aminoglycoside.
- An antibiotic called chloramphenicol (used to treat infections, particularly of the eyes).
Pregnancy and breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
The doctor will consider the benefit of treating you with C-Tri against the risk to your baby.
Driving and using machines
C-Tri can cause dizziness. If you feel dizzy, do not drive or use any tools or machines. Talk to your doctor if you experience these symptoms.
3. How C-Tri is given
C-Tri is usually given by a doctor or nurse. It can be given as
- a drip (intravenous infusion) or as an injection directly into a vein or
- into a muscle.
C-Tri is made up by the doctor, pharmacist or nurse and will not be mixed with or given to you at the same time as calcium-containing injections.
The usual dose
Your doctor will decide the correct dose of C-Tri for you. The dose will depend on the severity and type of infection; whether you are on any other antibiotics; your weight and age; how well your kidneys and liver are working. The number of days or weeks that you are given C-Tri depends on what sort of infection you have.
Adults, older people and children aged 12 years and over with a body weight greater than or equal to 50 kilograms (kg):
1 to 2 g once a day depending on the severity and type of infection. If you have a severe infection, your doctor will give you a higher dose (up to 4 g once a day). If your daily dose is higher than 2 g, you may receive it as a single dose once a day or as two separate doses.
Newborn babies, infants and children aged 15 days to 12 years with a body weight of less than 50 kg:
- 50-80 mg C-Tri for each kg of the child’s body weight once a day depending on the severity and type of infection. If you have a severe infection, your doctor will give you a higher dose up to 100 mg for each kg of body weight to a maximum of 4 g once a day. If your daily dose is higher than 2 g, you may receive it as a single dose once a day or as two separate doses.
- Children with a body weight of 50 kg or more should be given the usual adult dose.
Newborn babies (0-14 days)
- 20 – 50 mg C-Tri for each kg of the child’s body weight once a day depending on the severity and type of infection.
- The maximum daily dose is not to be more than 50 mg for each kg of the baby’s weight.
People with liver and kidney problems
You may be given a different dose to the usual dose. Your doctor will decide how much C-Tri you will need and will check you closely depending on the severity of the liver and kidney disease.
If you are given more C-Tri than you should
If you accidentally receive more than your prescribed dose, contact your doctor or nearest hospital straight away.
If you forget to use C-Tri
If you miss an injection, you should have it as soon as possible. However, if it is almost time for your next injection, skip the missed injection. Do not take a double dose (two injections at the same time) to make up for a missed dose.
If you stop using C-Tri
Do not stop taking C-Tri unless your doctor tells you to. If you have any further questions on the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine:
Treatment with ceftriaxone, particularly in elderly patients with serious kidney or nervous system problems may rarely cause decreased consciousness, abnormal movements, agitation and convulsions.
Severe allergic reactions (not known, frequency cannot be estimated from the available data) If you have a severe allergic reaction, tell a doctor straight away.
The signs may include:
- Sudden swelling of the face, throat, lips or mouth. This can make it difficult to breathe or swallow.
- Sudden swelling of the hands, feet and ankles.
Severe skin reactions (not known, frequency cannot be estimated from the available data) If you get a severe skin reaction, tell a doctor straight away.
The signs may include:
- A severe rash that develops quickly, with blisters or peeling of the skin and possibly blisters in the mouth (Stevens-Johnson syndrome and toxic epidermal necrolysis which are also known as SJS and TEN).
- A combination of any of the following symptoms: widespread rash, high body temperature, liver enzyme elevations, blood abnormalities (eosinophilia), enlarged lymph nodes and other body organs involvement (Drug Reaction with Eosinophilia and Systemic Symptoms which is also known as DRESS or drug hypersensitivity syndrome).
- Jarisch-Herxheimer reaction which causes fever, chills, headache, muscle pain, and skin rash that is usually self-limiting. This occurs shortly after starting C-Tri treatment for infections with spirochete such as Lyme disease.
Other possible side effects:
Common (may affect up to 1 in 10 people)
Abnormalities with your white blood cells (such as a decrease of leucocytes and an increase of eosinophils) and platelets (decrease of thrombocytes).
Loose stools or diarrhoea.
Changes in the results of blood tests for liver functions.
Rash.
Uncommon (may affect up to 1 in 100 people)
Fungal infections (for example, thrush or genital fungal infections).
A decrease in the number of white blood cells (granulocytopenia).
Reduction in number of red blood cells (anaemia).
Problems with the way your blood clots.
The signs may include bruising easily and pain and swelling of your joints.
Headache. Dizziness.
Feeling sick or being sick. Pruritis (itching).
Pain or a burning feeling along the vein where C-Tri has been given. Blisters, deep redness or rash, burned areas, pain, irritation, itching at the injection site.
A high temperature (fever).
Abnormal kidney function test (blood creatinine increased).
Rare (may affect up to 1 in 1,000 people)
Inflammation of the large bowel (colon). The signs include diarrhoea, usually with blood and mucus, stomach pain and fever.
Difficulty in breathing (bronchospasm).
A lumpy rash (hives) that may cover a lot of your body, feeling itchy and swelling.
Blood or sugar in your urine.
Oedema (fluid build-up).
Shivering.
Not known (Frequency cannot be estimated from the available data)
A secondary infection that may not respond to the antibiotic previously prescribed
Form of anaemia where red blood cells are destroyed (haemolytic anaemia).
Severe decrease in white blood cells (agranulocytosis).
Convulsions.
Vertigo (spinning sensation).
Inflammation of the pancreas (pancreatitis).
The signs include severe pain in the stomach which spreads to your back. Inflammation of the mucus lining of the mouth (stomatitis).
Inflammation of the tongue (glossitis).
The signs include swelling, redness and soreness of the tongue.
Problems with your gallbladder and/or liver which may cause pain, nausea, vomiting, yellowing of the skin, itching, unusually dark urine and clay coloured stools.
A neurological condition that may occur in neonates with severe jaundice (kernicterus).
Kidney problems caused by deposits of calcium ceftriaxone.
There may be pain when passing water (urine) or low output of urine.
A false positive result in a Coombs’ test (a test for some blood problems).
A false positive result for galactosaemia (an abnormal build up of the sugar galactose).
C-Tri may interfere with some types of blood glucose tests - please check with your doctor.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store C-Tri
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the vial or bottle label after EXP. The expiry date refers to the last day of that month.
Do not store above 30°C, keep vial or bottle in the outer carton in order to protect from light.
Chemical and physical in-use stability of the reconstituted product has been demonstrated for at least 6 hours at or below 25°C or 24 hours at 2-8°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would not be longer than the times stated above for the chemical and physical in-use stability. Do not throw away any medicines via wastewater. Ask your pharmacist to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What C-Tri contains
Each vial contains:
Ceftriaxone Sodium IP
equivalent to Ceftriaxone 125 mg / 250 mg / 500 mg / 1.0 g / 2.0 g
This pack contains Sterile Water for Injections (SWFI) IP 5/5/5/10/10 ml.
Packing information:
C-Tri is available in packs of 1 vial.
C Tri 125 contains a vial of 125 mg with SWFI IP 5 ml.
C Tri 250 contains a vial of 250 mg with SWFI IP 5 ml.
C Tri 500 contains a vial of 500 mg with SWFI IP 5 ml.
C Tri 1.0 g contains a vial of 1.0 g with SWFI IP 10 ml.
C Tri 2.0 g contains a vial of 2.0 g with SWFI IP 10 ml.
Marketing Authorisation Holder
Zuventus Heathcare Ltd.
Kamerey Bhasmay, Elaka Pakyong, Rangpo,
East-Sikkim 737 132, India