Diof Suspension
1.0 Generic name
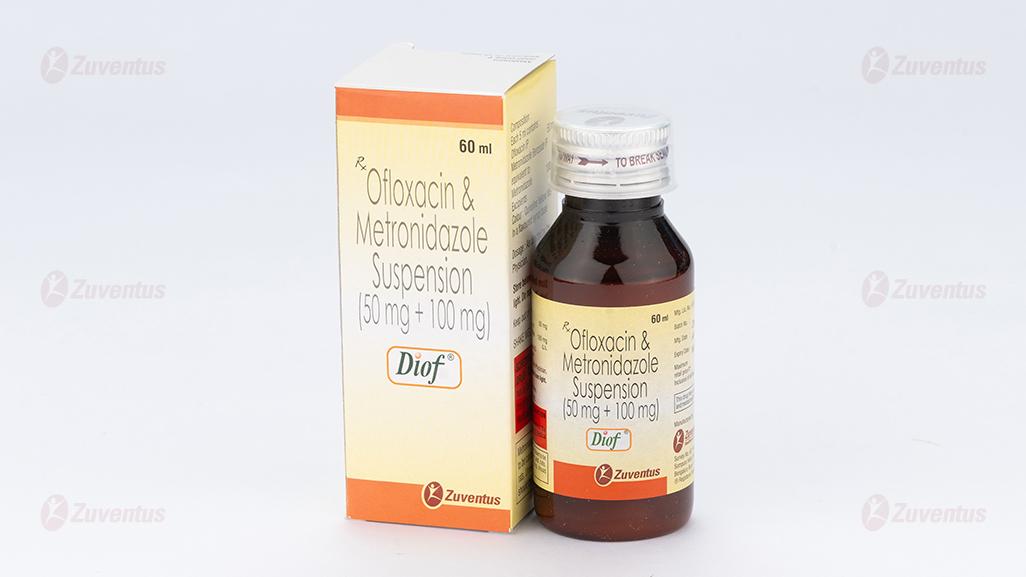
Ofloxacin & Metronidazole Suspension
2.0 Qualitative and quantitative composition
Diof
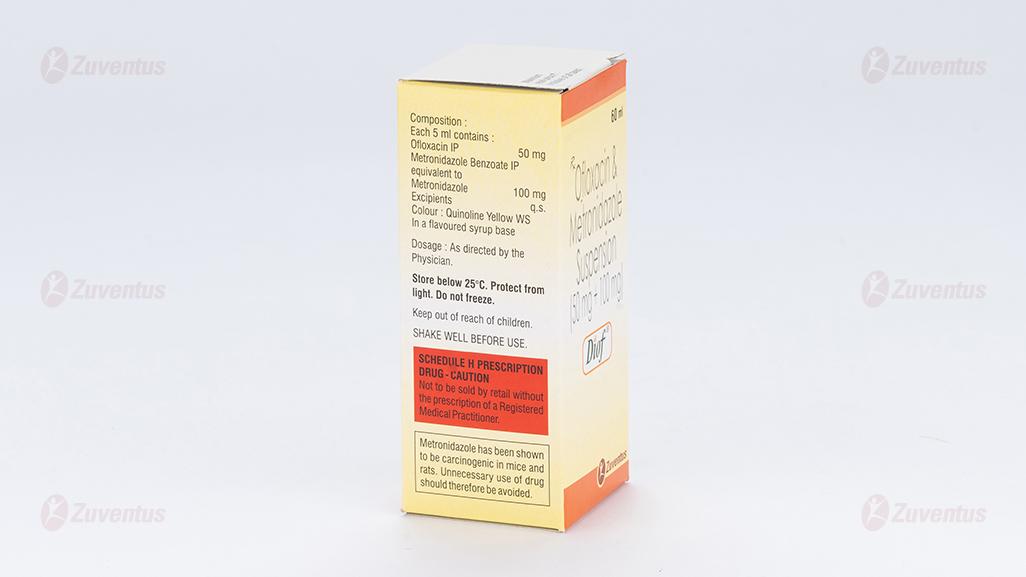
Each 5 ml contains :
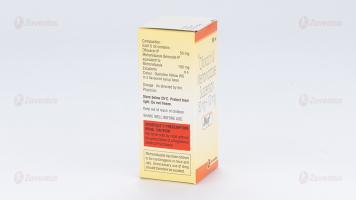
Ofloxacin IP 50 mg
Metronidazole Benzoate IP
equivalent to Metronidazole 100 mg
Excipients q.s.
Colour : Quinoline Yellow WS
In a flavoured syrup base
Diof-DS
Each 5 ml contains :
Ofloxacin IP 100 mg
Metronidazole Benzoate IP
equivalent to Metronidazole 200 mg
Excipients q.s.
Colour : Quinoline Yellow WS
In a flavoured syrup base
3.0 Dosage form and strength
Suspension, Ofloxacin 50 mg / 100 mg and Metronidazole 100 mg / 200 mg
4.0 Clinical particulars
4.1 Therapeutic indication
For the treatment of diarrhea of mixed infection in adult patients only.
4.2 Posology and method of administration
Dose of Ofloxacin is 15 mg/kg/day and that of Metronidazole is 30 mg/kg/day in two divided doses. Therefore, New Normet suspension containing 100 mg of Ofloxacin and 200 mg of Metronidazole in each 5 ml is an appropriate combination.
Dosage: As advised by the physician.
Geriatric Use
- Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as ofloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involves the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing ofloxacin to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue ofloxacin and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur.
- In elderly geriatric patients, monitoring for metronidazole associated adverse events is recommended. Decreased liver function in geriatric patients can result in increased concentrations of metronidazole that may necessitate adjustment of metronidazole dosage.
Pediatric Use
- Safety and effectiveness in pediatric patients and adolescents below the age of 18 years have not been established. Ofloxacin causes arthropathy (arthrosis) and osteochondrosis in juvenile animals of several species.
- Safety and effectiveness of metronidazole in pediatric patients have not been established, except for the treatment of amebiasis.
4.3 Contraindications
- Patients hypersensitivity to any fluoroquinolone antibacterials.
- Patients having history of epilepsy or tendon disorders or CNS disorder with a lowered seizure threshold should be avoided giving ofloxacin formulation.
- Ofloxacin shall not be prescribed to Adolescent, pregnant or breastfeeding women, since animal experiments do not entirely exclude the risk of damage to the growth-plate cartilage.
- In patients with latent or actual defects in glucose-6-phosphate dehydrogenase activity because they more prone to haemolytic reactions when treated with quinolone antibacterial agents.
- Ofloxacin is not the drug of first choice in pneumonia, caused by Streptococcus pneumoniae or Chlamydia pneumonia, Methicillin resistant S. aureus. Ofloxacin is not recommended for the treatment of known or suspected MRSA infections unless laboratory results have confirmed susceptibility of the organism to ofloxacin.
- Metronidazole is contraindicated in patients with a prior history of hypersensitivity to metronidazole or other nitroimidazole derivatives.
- In patients with trichomoniasis, metronidazole is contraindicated during the first trimester of pregnancy.
- Use of metronidazole is associated with psychotic reactions in alcoholic patients who were using disulfiram concurrently. Do not administer metronidazole to patients who have taken disulfiram within the last two weeks.
- Use of oral metronidazole is associated with a disulfiram-like reaction to alcohol, including abdominal cramps, nausea, vomiting, headaches, and flushing. Discontinue consumption of alcohol or products containing propylene glycol during and for at least three days after therapy with metronidazole.
4.4 Special warnings and precautions for use
Ofloxacin
Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitisand Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects:
Fluoroquinolones, including ofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting ofloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions. Discontinue ofloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including ofloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
Tendinopathy and Tendon Rupture: Fluoroquinolones, including ofloxacin, have been associated with an increased risk of tendinitis and tendon rupture in all ages. Discontinue ofloxacin immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon. Avoid fluoroquinolones, including ofloxacin, in patients who have a history of tendon disorders or have experienced tendinitis or tendon rupture. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug. Peripheral Neuropathy: Fluoroquinolones, including ofloxacin, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including ofloxacin. Symptoms may occur soon after initiation of norfloxacin and may be irreversible in some patients. Discontinue ooxacin immediately if the patient experiences symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness, or other alterations in sensations including light touch, pain, temperature, position sense and vibratory sensation, and/or motor strength in order to minimize the development of an irreversible condition. Avoid fluoroquinolones, including ofloxacin, in patients who have previously experienced peripheral neuropathy.
Central Nervous System Effects: Psychiatric Adverse Reactions: Fluoroquinolones, including ooxacin, have been associated with an increased risk of psychiatric adverse reactions, including toxic psychoses or hallucinations; agitation; delirium, confusion, disorientation, or disturbances in attention; nervousness or restlessness, memory impairment. If these reactions occur in patients receiving ofloxacin, the drug should be discontinued and appropriate measures instituted.
Central Nervous System Adverse Reactions: Fluoroquinolones have been associated with an increased risk of seizures (convulsions), increased intracranial pressure (pseudotumor cerebri), lightheadedness, or tremors.The effects of ofloxacin on brain function or on the electrical activity of the brain have not been tested. Therefore, until more information becomes available, ofloxacin, like all other quinolones, should be used with caution in patients with known or suspected CNS disorders, such as severe cerebral arteriosclerosis, epilepsy, and other factors which predispose to seizures. Exacerbation of Myasthenia Gravis: Fluoroquinolones, including ofloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Postmarketing serious adverse events, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid ofloxcain in patients with known history of myasthenia gravis. The safety and efficacy of ofloxacin in pediatric patients and adolescents (under the age of 18 years), pregnant women, and lactating women have not been established.
Hypersensitivity Reactions: Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with quinolones, including ofloxacin. These reactions often occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), dyspnea, urticaria, itching, and other serious skin reactions. This drug should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous incluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated. Other serious and sometimes fatal events, some due to hypersensitivity, and
some due to uncertain etiology, have been reported in patients receiving therapy with quinolones, including ofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- fever, rash, severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome);
- vasculitis; arthralgia; myalgia; serum sickness;
- allergic pneumonitis;
- interstitial nephritis; acute renal insufficiency or failure;
- hepatitis; jaundice; acute hepatic necrosis or failure;
- anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
The drug should be discontinued immediately at the first appearance of a skin rash, jaundice or any other sign of hypersensitivity and supportive measures instituted.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ofloxacin tablets, and may range in severity from mild diarrhea to fatal colitis. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
General:
- Prescribing ofloxacin tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
- Adequate hydration of patients receiving ofloxacin should be maintained to prevent the formation of a highly concentrated urine.
- Administer ofloxacin with caution in the presence of renal or hepatic insufficiency/impairment. In patients with known or suspected renal or hepatic insufficiency/impairment, careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of ofloxacin may be reduced. In patients with impaired renal function (creatinine clearance ≤ 50 mg/mL), alteration of the dosage regimen is necessary.
- Moderate to severe photosensitivity/phototoxicity reactions, the latter of which may manifest as exaggerated sunburn reactions (e.g., burning, erythema, exudation, vesicles, blistering, edema) involving areas exposed to light (typically the face, “V” area of the neck, extensor surfaces of the forearms, dorsa of the hands), can be associated with the use of quinolones after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided. Drug therapy should be discontinued if photosensitivity/phototoxicity occurs.
- As with other quinolones, ofloxacin should be used with caution in any patient with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction).
- A possible interaction between oral hypoglycemic drugs (e.g., glyburide/glibenclamide) or with insulin and fluoroquinolone antimicrobial agents have been reported resulting in a potentiation of the hypoglycemic action of these drugs, sometimes resulting in coma or death. The mechanism for this interaction is not known. If a hypoglycemic reaction occurs in a patient being treated with ofloxacin, discontinue ofloxacin immediately and consult a physician. As with any potent drug, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during prolonged therapy.
Torsades de pointes
Some quinolones, including ofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Rare cases of torsades de pointes have been spontaneously reported during post-marketing surveillance in patients receiving quinolones, including ofloxacin. Ofloxacin should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving class IA (quinidine, procainamide), or class III (amiodarone, sotalol) antiarrhythmic agents.
Metronidazole
- Regular clinical and laboratory monitoring (especially leucocyte count) are advised if administration of Metronidazole for more than 10 days is considered to be necessary and patients should be monitored for adverse reactions, such as peripheral or central neuropathy (such as paraesthesia, ataxia, dizziness, convulsive seizures).
- Metronidazole should be used with caution in patients with active or chronic severe peripheral and central nervous system disease due to the risk of neurological aggravation.
- Cases of severe hepatotoxicity/acute hepatic failure, including cases with a fatal outcome with very rapid onset after treatment initiation in patients with Cockayne syndrome have been reported with products containing metronidazole for systemic use. In this population, metronidazole should therefore be used after careful benefit-risk assessment and only if no alternative treatment is available. Liver function tests must be performed just prior to the start of therapy, throughout and after end of treatment until liver function is within normal ranges, or until the baseline values are reached. If the liver function tests become markedly elevated during treatment, the drug should be discontinued.
- Patients with Cockayne syndrome should be advised to immediately report any symptoms of potential liver injury to their physician and stop taking metronidazole.
- Cases of severe bullous skin reactions such as Stevens Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) or acute generalised exanthematous pustulosis (AGEP) have been reported with metronidazole. If symptoms or signs of SJS, TEN or AGEP are present, Metronidazole treatment must be immediately discontinued.
- Metronidazole is mainly metabolised by hepatic oxidation. Substantial impairment of metronidazole clearance may occur in the presence of advanced hepatic insufficiency. Significant cumulation may occur in patients with hepatic encephalopathy and the resulting high plasma concentrations of metronidazole may contribute to the symptoms of the encephalopathy. Metronidazole should therefore, be administered with caution to patients with hepatic encephalopathy. The daily dosage should be reduced to one third and may be administered once daily.
Psychotic Reaction with Disulfiram
Use of oral metronidazole is associated with psychotic reactions in alcoholic patients who were using disulfiram concurrently. Do not administer metronidazole to patients who have taken disulfiram within the last two weeks.
Interaction with Alcohol
Use of oral metronidazole is associated with a disulfiram-like reaction to alcohol, including abdominal cramps, nausea, vomiting, headache, flushing, throbbing headache, rapid heartbeat, palpitation, sweating, thirst, chest pain, lightheadness, blurred vision, dizziness and difficulty in breathing. Rarely more severe reactions may include abnormal heart rhythm, heart attack, heart failure, unconsciousness, convulsions and even death. Discontinue consumption of alcohol or products containing propylene glycol during and for at least three days after therapy with metronidazole.
Central and Peripheral Nervous System Effects
Encephalopathy and peripheral neuropathy: Cases of encephalopathy and peripheral neuropathy (including optic neuropathy) have been reported with metronidazole. Encephalopathy has been reported in association with cerebellar toxicity characterized by ataxia, dizziness, and dysarthria. CNS lesions seen on MRI have been described in reports of encephalopathy. CNS symptoms are generally reversible within days to weeks upon discontinuation of metronidazole. CNS lesions seen on MRI have also been described as reversible. Peripheral neuropathy, mainly of sensory type has been reported and is characterized by numbness or paresthesia of an extremity. Convulsive seizures have been reported in patients treated with metronidazole. Aseptic meningitis: Cases of aseptic meningitis have been reported with metronidazole. Symptoms can occur within hours of dose administration and generally resolve after metronidazole therapy is discontinued. The appearance of abnormal neurologic signs and symptoms demands the prompt evaluation of the benefit/risk ratio of the continuation of therapy.
Hepatic Impairment
Patients with hepatic impairment metabolize metronidazole slowly, with resultant accumulation of metronidazole in the plasma. For patients with severe hepatic impairment (Child-Pugh C), a reduced dose of metronidazole is recommended. For patients with mild to moderate hepatic impairment, no dosage adjustment is needed but these patients should be monitored for metronidazole associated adverse events.
Renal Impairment
Patients with end-stage renal disease may excrete metronidazole and metabolites slowly in the urine, resulting in significant accumulation of metronidazole metabolites. Monitoring for metronidazole associated adverse events is recommended.
Fungal Superinfections
Known or previously unrecognized candidiasis may present more prominent symptoms during therapy with metronidazole and requires treatment with a candidacidal agent.
Use in Patients with Blood Dyscrasias
Metronidazole is a nitroimidazole and should be used with caution in patients with evidence of or history of blood dyscrasia. A mild leukopenia has been observed during its administration; however, no persistent hematologic abnormalities attributable to metronidazole have been observed in clinical studies. Total and differential leukocyte counts are recommended before and after therapy.
Drug-Resistant Bacteria and Parasites
Prescribing metronidazole in the absence of a proven or strongly suspected bacterial or parasitic infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria and parasites.
4.5 Drugs interactions
Ofloxacin
Antacids, Sucralfate, Metal Cations, Multivitamins
Quinolones form chelates with alkaline earth and transition metal cations. Administration of quinolones with antacids containing calcium, magnesium, or aluminum, with sucralfate, with divalent or trivalent cations such as iron, or with multivitamins containing zinc or with didanosine, may substantially interfere with the absorption of quinolones resulting in systemic levels considerably lower than desired. These agents should not be taken within the two-hour period before or within the twohour period after ofloxacin administration.
Caffeine
Interactions between ofloxacin and caffeine have not been detected.
Cimetidine
Cimetidine has demonstrated interference with the elimination of some quinolones. This interference has resulted in significant increases in half-life and AUC of some quinolones. The potential for interaction between ofloxacin and cimetidine has not been studied.
Cyclosporine
Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with some other quinolones. The potential for interaction between ofloxacin and cyclosporine has not been studied.
Drugs Metabolized by Cytochrome P450 Enzymes
Most quinolone antimicrobial drugs inhibit cytochrome P450 enzyme activity. This may result in a prolonged half-life for some drugs that are also metabolized by this system (e.g., cyclosporine, theophylline/methylxanthines, warfarin) when co-administered with quinolones. The extent of this inhibition varies among different quinolones.
Non-Steroidal Anti-Inflammatory Drugs
The concomitant administration of a non-steroidal anti-inflammatory drug with a quinolone, including ofloxacin, may increase the risk of CNS stimulation and convulsive seizures.
Probenecid
The concomitant use of probenecid with certain other quinolones has been reported to affect renal tubular secretion. The effect of probenecid on the elimination of ofloxacin has not been studied.
Theophylline
Steady-state theophylline levels may increase when ofloxacin and theophylline are administered concurrently. As with other quinolones, concomitant administration of ofloxacin may prolong the half life of theophylline, elevate serum theophylline levels, and increase the risk of theophylline-related adverse reactions. Theophylline levels should be closely monitored and theophylline dosage adjustments made, if appropriate, when ofloxacin is co-administered. Adverse reactions (including seizures) may occur with or without an elevation in the serum theophylline level.
Warfarin
Some quinolones have been reported to enhance the effects of the oral anticoagulant warfarin or its derivatives. Therefore, if a quinolone antimicrobial is administered concomitantly with warfarin or its derivatives, the prothrombin time or other suitable coagulation test should be closely monitored.
Antidiabetic Agents (e.g., insulin, glyburide/glibenclamide)
Since disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concurrently with quinolones and an antidiabetic agent, careful monitoring of blood glucose is recommended when these agents are used concomitantly.
Interaction with Laboratory or Diagnostic Testing
Some quinolones, including ofloxacin, may produce false-positive urine screening results for opiates using commercially available immunoassay kits. Confirmation of positive opiate screens by more specific methods may be necessary.
Metronidazole
Disulfiram
Psychotic reactions have been reported in alcoholic patients who are using metronidazole and disulfiram concurrently. Metronidazole should not be given to patients who have taken disulfiram within the last two weeks.
Alcoholic Beverages
Abdominal cramps, nausea, vomiting, headaches, and flushing may occur if alcoholic beverages or products containing propylene glycol are consumed during or following metronidazole therapy.
Warfarin and other Oral Anticoagulants
Metronidazole has been reported to potentiate the anticoagulant effect of warfarin and other oral coumarin anticoagulants, resulting in a prolongation of prothrombin time. When FLAGYL is prescribed for patients on this type of anticoagulant therapy, prothrombin time and INR should be carefully monitored.
Lithium
In patients stabilized on relatively high doses of lithium, short-term metronidazole therapy has been associated with elevation of serum lithium and, in a few cases, signs of lithium toxicity. Serum lithium and serum creatinine levels should be obtained several days after beginning metronidazole to detect any increase that may precede clinical symptoms of lithium intoxication.
Busulfan
Metronidazole has been reported to increase plasma concentrations of busulfan, which can result in an increased risk for serious busulfan toxicity. Metronidazole should not be administered concomitantly with busulfan unless the benefit outweighs the risk. If no therapeutic alternatives to metronidazole are available, and concomitant administration with busulfan is medically needed, frequent monitoring of busulfan plasma concentration should be performed and the busulfan dose should be adjusted accordingly.
Drugs that Inhibit CYP450 Enzymes
The simultaneous administration of drugs that decrease microsomal liver enzyme activity, such as cimetidine, may prolong the half-life and decrease plasma clearance of metronidazole.
Drugs that Induce CYP450 Enzymes
The simultaneous administration of drugs that induce microsomal liver enzymes, such as phenytoin or phenobarbital, may accelerate the elimination of metronidazole, resulting in reduced plasma levels; impaired clearance of phenytoin has also been reported.
Drug/Laboratory Test Interactions
Metronidazole may interfere with certain types of determinations of serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides, and glucose hexokinase. Values of zero may be observed. All of the assays in which interference has been reported involve enzymatic coupling of the assay to oxidation-reduction of nicotinamide adenine dinucleotide (NAD+ NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7.
4.6 Use in special populations
Pregnancy
- There are, however, no adequate and well-controlled studies in pregnant women. Ofloxacin should be used during pregnancy only if the potential benefit justies the potential risk to the foetus.
- There are no adequate and well controlled studies of Metronidazole in pregnant women. Metronidazole crosses the placental barrier and its effects on the human fetal organogenesis are not known. Reproduction studies have been performed in rats, rabbits, and mice at doses similar to the maximum recommended human dose based on body surface area comparisons. There was no evidence of harm to the foetus due to metronidazole.
Nursing Mothers
- Because of the potential for serious adverse reactions from ooxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
- Metronidazole is present in human milk at concentrations similar to maternal serum levels, and infant serum levels can be close to or comparable to infant therapeutic levels. Because of the potential for tumorigenicity shown for metronidazole in mouse and rat studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Alternatively, a nursing mother may choose to pump and discard human milk for the duration of metronidazole therapy, and for 24 hours after therapy ends and feed her infant stored human milk or formula.
4.7 Effects on ability to drive and use machines
Since there have been occasional reports of drowsiness/somnolence, impairment of skills, dizziness/vertigo and visual disturbances, which may impair the patient's ability to concentrate and react, and therefore may constitute a risk in situations where these abilities are of special importance (e.g. driving a car or operating machinery); patients should advised not to drive or operate machinery if these symptoms occur. These effects may be enhanced by alcohol.
4.8 Undesirable effects
Ofloxacin
In clinical trials, the following events were considered likely to be drug-related in patients receiving multiple doses of ofloxacin: nausea, insomnia, headache, dizziness, diarrhea, vomiting, rash, pruritus, external genital pruritus in women, vaginitis, dysgeusia.
In clinical trials, the most frequently reported adverse events, regardless of relationship to drug, were: nausea, headache, insomnia, external genital pruritus in women, dizziness, vaginitis, diarrhea, vomiting.
In clinical trials, the following events, regardless of relationship to drug, occurred in 1 to 3% of patients: abdominal pain and cramps, chest pain, decreased appetite, dry mouth, dysgeusia, fatigue, fatulence, gastrointestinal distress, nervousness, pharyngitis, pruritus, fever, rash, sleep disorders, somnolence, trunk pain, vaginal discharge, visual disturbances, and constipation.
Additional events, occurring in clinical trials at a rate of less than 1%, regardless of relationship to drug, were :

The following laboratory abnormalities appeared in ≥1% of patients receiving multiple doses of ofloxacin. It is not known whether these abnormalities were caused by the drug or the underlying conditions being treated.

In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with other quinolones. The relationship of the drugs to these events is not presently established.
Metronidazole


Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.co.in/drug-safety-reporting
4.9 Overdose
Symptoms-The most important signs to be expected following acute overdose are CNS symptoms such as confusion, dizziness, impairment of consciousness and convulsive seizures increases in QT interval as well as gastrointestinal reactions such as nausea and mucosal erosions.
Management-In the case of overdose steps to remove any unabsorbed ofloxacin e.g. gastric lavage, administration of adsorbants and sodium sulphate, if possible during the first 30 minutes, are recommended; antacids are recommended for protection of the gastric mucosa. ECG monitoring should be undertaken, because of the possibility of QT interval prolongation. Antacids may be used for protection of gastric mucosa. No specific antidote exists. In cases of suspected massive overdose, symptomatic and supportive treatment should be instituted.
5.0 Pharmacological properties
5.1 Mechanism of Action
Ofloxacin inhibits bacterial DNA replication by inhibiting bacterial topoisomerases, particularly DNA gyrase and topoisomerase IV. The nitro group of metronidazole acts as electron acceptor and is thus reduced to a chemically reactive drug form. This produces biochemical lesions in the cells, thus causing death. The major site of action is believed to be DNA, where it causes loss of the helical structure and inhibits synthesis.
5.2 Pharmacodynamic properties
Ofloxacin
Therapeutic doses of ofloxacin are devoid of pharmacological effects on the voluntary or autonomic nervous system. According to DIN 58 940, the following limits apply for ofloxacin:
Susceptible (S)≤ 1 mg/L, Intermediate (I) = 2 mg/L, Resistant (R) ≥ 4 mg/L.
Range of acquired bacterial resistance to ooxacin is as follows:
Normally susceptible
Aerobic Gram-positive micro-organisms: S. aureus - methicillin-sensitive (0.3-12.6%), S. pyogenes (2-5%) Aerobic Gram-negative micro-organisms: Acinetobacter spp (0.3-7.3%), Citrobacter spp. (3-15%), Enterobacter spp. (2-13%), E. coli (1-8%), H. influenzae (1%), Klebsiella spp. (1-10%), Moraxella spp. (0-0.2%), Morganella morganii (0- 6.9%), N. gonorrhoeae (25%) Proteus spp. (1-15%), Serratia marcescens (2-2.4%)
Others: Chlamydia spp, L. pneumophila
Intermediately susceptible
Aerobic Gram-positive micro-organisms: S. pneumoniae (70%), Providentia (17.1%)
Aerobic Gram-negative micro-organisms: E. faecalis (50%), P. aeruginosa (20-30%), Serratia spp. (20-40%)
Metronidazole
A potential for development of resistance exists against metronidazole. Resistance may be due to multiple mechanisms that include decreased uptake of the drug, altered reduction efficiency, overexpression of the efflux pumps, inactivation of the drug, and/or increased DNA damage repair.
Activity in Vitro and in Clinical Infections
- Gram-positive anaerobes: Clostridium species, Eubacterium species, Peptococcus species, Peptostreptococcus species.
- Gram-negative anaerobes: Bacteroides fragilis group (B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B. vulgatus), Fusobacterium species.
Metronidazole exhibits in vitro minimal inhibitory concentrations (MIC’s) of 8 mcg/mL or less against most (≥ 90%) isolates of the following bacteria; however, the safety and effectiveness of metronidazole in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
- Gram-negative anaerobes: Bacteroides fragilis group (B. caccae, B. uniformis), Prevotella species (P. bivia, P. buccae, P. disiens)
5.3 Pharmacokinetic properties

6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Ofloxacin
Preclinical effects in conventional studies of safety pharmacology, acute toxicity, repeated dose toxicity, reproductive studies were observed only at exposures considered sufficiently in excess of the maximum human exposure indicating little relevance to clinical use. Joint toxicity was observed at exposure in the human therapeutic range in juvenile rats and dogs. Ofloxacin exhibits a neurotoxic potential and causes reversible testicular alterations at high doses.
Mutagenicity studies showed no evidence for mutagenicity of ofloxacin. However, like some other quinolones Ofloxacin is phototoxic in animals at exposure in the human therapeutic range. The phototoxic, photomutagenic and photocarcinogenic potential of ofloxacin is comparable with that of other gyrase inhibitors. Preclinical data from conventional genotoxicity studies reveal no special hazard to humans, carcinogen potential has not been investigated.
Reproduction toxicity
Ofloxacin has no effect on fertility, peri- or postnatal development, and therapeutic doses did not lead to any teratogenic or other embryotoxic effects in animals. Ooxacin crosses the placenta and levels reached in the amniotic fluid are about 30% of the maximal concentrations measured in maternal serum.
Metronidazole
Metronidazole has been shown to be carcinogenic in the mouse and in the rat following chronic oral administration however similar studies in the hamster have given negative results. Epidemiological studies have provided no clear evidence of an increased carcinogenic risk in humans. Metronidazole has been shown to be mutagenic in bacteria in vitro. In studies conducted in mammalian cells in vitro as well as in rodent or humans in vivo, there was inadequate evidence of a mutagenic effect of metronidazole, with some studies reporting mutagenic effects, while others studies were negative.
7.0 Description
Ofloxacin is a synthetic broad-spectrum second-generation fluoroquinolone antibacterial agent. Metronidazole Benzoate is the benzoate ester of metronidazole, a synthetic nitroimidazole derivative with antiprotozoal and antibacterial activities.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
Diof : A bottle of 60 ml.
Diof-DS : A bottle of 60 ml.
8.4 Storage and handling instructions
Store below 25°C. Protect from light. Do not freeze.
Keep out of reach of children.
Shake well before use.
9.0 Patient counselling information
- Your medicine contains Ofloxacin and Metronidazole which belong to a group of medicines called antibacterials.
- Do not take this medicine, if you are allergic to ofloxacin or metronidazole.
Signs of an allergic reaction include a rash, itching or shortness of breath.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
- If you have any further questions, ask your doctor or pharmacist.
12.0 Date of revision
19 July 2024