Efnocar T 20-40 Tablets
Therapy Area
Cardiology
1.0 Generic name
Efonidipine Hydrochloride Ethanolate and Telmisartan Tablets
2.0 Quantitative and qualitative composition
Efnocar T 20/40
Each uncoated bilayered tablet contains :
Efonidipine Hydrochloride Ethanolate 20 mg
Telmisartan IP 40 mg
Excipients q.s.
Efnocar T 40/40
Each uncoated bilayered tablet contains :
Efonidipine Hydrochloride Ethanolate 40 mg
Telmisartan IP 40 mg
Excipients q.s.
3.0 Dosage form and strength
Tablet Efonidipine Hydrochloride [20/40 mg] and Telmisartan [40/40 mg]
4.0 Clinical particulars
4.1 Therapeutic indication
For the management of stage II hypertension.
4.2 Posology and method of administration
Adult
The usual recommended dose is 1 tablet daily. It may be increased to 2 tablets per day depending upon the blood pressure control or as directed by the Physician.
Efonidipine
Essential hypertension : 20 - 40 mg orally once daily. A dose of up to 80 mg/day has been reported to be safe and effective in clinical trials.
Telmisartan
Essential hypertension : The usually effective dose is 40 mg once daily. In cases where the target blood pressure is not achieved, the dose of Telmisartan can be increased to a maximum of 80 mg once daily.
Use in special populations
Pediatric
The combination of Efonidipine and Telmisartan is not recommended in infants and children.
Geriatric
Efonidipine should be started at low dose (20 mg/day) in elderly. Patient should be carefully observed for development of hypotension. Dose may be halved if there is intolerance to the 20 mg/day dosage regimen. The pharmacokinetics of Telmisartan does not differ in young and elderly patients.
Hepatic impairment
Pharmacokinetic studies in patients with hepatic impairment showed an increase in absolute bioavailability of Telmisartan up to nearly 100%. Patients with hepatic impairment should be carefully monitored and dose should be slowly up titrated. Hence, caution should be exercised while administering FDC of Efonidipine and Telmisartan in patients with mild to moderate hepatic impairment.
Renal impairment
In Telmisartan limited experience is available in patients with severe renal impairment or haemodialysis. A lower starting dose of 20 mg is recommended in these patients. No dose adjustment is required for patients with mild to moderate renal impairment. Hence, caution should be exercised while administering FDC of Efonidipine and Telmisartan in patients with severe renal impairment or haemodialysis.
4.3 Contraindications
The combination of Efonidipine and Telmisartan is contraindicated in the following situations :
- Known hypersensitivity to the active substance Efonidipine and Telmisartan or any other component of the formulation.
- In pregnant women
- Severe hepatic impairment.
- Concomitant use of ACE inhibitors
4.4 Warning and precautions
Should be administered with caution in patients with hepatic impairment.
The drug may worsen clinical condition in patients with sinus bradycardia, sinus arrest or sinus node dysfunction.
Should not be taken with grapefruit juice as there may be excessive lowering of blood pressure.
Administration of the drug may cause hypotension. Under such circumstance appropriate measures should be taken to either reduce the dose or withdraw the drug
Dizziness may occur while taking the antihypertensive agents. Hence, working on aerial platform, working with machinery and/or driving should be avoided.
4.5 Drug interactions
Efonidipine
Concomitant administration of other antihypertensive agent/s may enhance the antihypertensive effect of Efonidipine.
Administration of Calcium channel blockers (CCBs) with cimetidine may cause elevated levels of CCBs.
Increased levels of CCBs observed when taken concomitantly with grapefruit juice which may result in excessive lowering of blood pressure.
Efonidipine when taken along with Tacrolimus may cause increased blood levels of Tacrolimus.
Telmisartan
- Concomitant administration of Digoxin may increase peak plasma concentration of Digoxin. When initiating, adjusting, and discontinuing Telmisartan, monitor Digoxin levels in order to maintain levels within the therapeutic range.
- Telmisartan may cause hyperkalaemia with other medicinal products acting on the renin-angiotensin-aldosterone system.
- Angiotensin II receptor antagonists such as Telmisartan, attenuate diuretic induced potassium loss.
- NSAIDs (i.e. Acetylsalicylic Acid at anti-inflammatory dosage regimens, COX-2 inhibitors and non-selective NSAIDs) may reduce the antihypertensive effect of angiotensin II receptor antagonists and increase risk of renal impairment.
- Co-administration of Telmisartan and Ramipril led to an increase of up to 2.5 fold in the AUC0-24 and Cmax of Ramipril and Ramiprilat.
- Prior treatment with high dose diuretics such as Furosemide (loop diuretic) and Hydrochlorothiazide (Thiazide diuretic) may result in volume depletion, and there is a risk of hypotension when initiating therapy with Telmisartan.
- The blood pressure lowering effect of Telmisartan can be increased by concomitant use of other antihypertensive medicinal products.
- Based on their pharmacological properties it can be expected that the following medicinal products may potentiate the hypotensive effects of all antihypertensives including Telmisartan : Baclofen, Amifostine. Furthermore, orthostatic hypotension may be aggravated by Alcohol, Barbiturates, Narcotics, or Antidepressants.
4.6 Special populations
Pregnancy
Efonidipine should not be administered to pregnant women and women suspected of being pregnant. Clinical experience with ACE inhibitors has shown increased risk of fetal and neonatal toxicity and death (“ACE inhibitor fetopathy”) when women are exposed to RAAS-active drugs during the last two trimesters of pregnancy. Since this may be a class effect of drugs acting on the RAAS, Telmisartan is contraindicated during pregnancy.
Lactation
Administration to lactating women should be avoided unless benefit significantly surpasses the risk to the child. Mothers on Efonidipine treatment should avoid breast feeding. It is not known whether Telmisartan is excreted in human milk, but Telmisartan was shown to be present in the milk of lactating rats. Because of the potential for adverse effects on the nursing infant, decide either drug or nursing should be discontinued, taking into account the importance of the drug to the mother.
4.7 Effects on ability to drive and use machines
Efonidipine / Telmisartan can have influence on the ability to drive and use machines. Dizziness or drowsiness may occasionally occur when taking Efonidipine/Telmisartan.
4.8 Undesirable effects
Efonidipine
Common side effects
The common side effects are hot flushes, palpitations, facial flushing and headache. In addition, elevation in serum total cholesterol, ALT (SGPT), AST (SGOT) and BUN may occur.
Uncommon side effects
Hepatobiliary disorders : LDH and alkaline phosphatase, Increase in bilirubin.
Renal and urinary disorders : serum creatinine rise, Proteinuria, Frequent urination.
Blood and lymphatic system disorders : Decreased hemoglobin, Decreased hematocrit, Decreased red blood cells,
Eosinophilia, Leukopenia, Thrombocytopenia.
Hypersensitivity : Rash, Itching
Cardiac disorders : Palpitations, Chest pain, Decreased blood pressure, Bradycardia, Tachycardia, Atrial fibrillation,
Premature ventricular contraction, Sick sinus syndrome, Atrioventricular junctional rhythm, Atrioventricular block, Shock
Nervous system disorders : Sluggishness and light-headedness, Drowsiness, Numbness
Gastrointestinal disorders : Nausea, Stomach discomfort, Abdominal pain, Vomiting, Constipation, Diarrhea, Gingival hyperplasia
Ear and labyrinth disorders : Tinnitus
Investigations : Increased CK (CPK), Increased uric acid and hypokalemia, Increased triglycerides
General : Malaise, Sweating, Edema
Telmisartan
Common side effects
The most frequent spontaneously reported events are Headache, Dizziness, Asthenia, Coughing, Nausea, Fatigue, Weakness, Edema, Face edema, Lower limb edema, Angioneurotic edema, Urticaria, Hypersensitivity, Sweating increased, Erythema, Chest pain, Atrial fibrillation, Congestive heart failure, Myocardial infarction, Blood pressure increased, Hypertension aggravated, Hypotension (including Postural hypotension), Hyperkalemia, Syncope, Dyspepsia, Diarrhea, Pain, Urinary tract infection, Erectile dysfunction, Back pain, Abdominal pain, Muscle cramps (including Leg cramps), Myalgia, Bradycardia, Eosinophilia, Thrombocytopenia, Uric acid increased, Abnormal hepatic function/liver disorder, Renal impairment including acute renal failure, Anemia, increased CPK, Anaphylactic reaction, Tendon pain (including Tendonitis, Tenosynovitis), Drug eruption (Toxic skin eruption mostly reported as Toxicoderma, Rash and Urticaria), Hypoglycemia (in Diabetic patients), and Angioedema (with Fatal outcome).
Uncommon side effects
Infections : Upper respiratory tract infection including pharyngitis and sinusitis, Sepsis
including fatal outcome
Psychiatric disorders : Insomnia, Depression, Anxiety
Nervous system disorders : Somnolence
Eye disorders : Visual disturbance
Ear and labyrinth disorders : Vertigo
Cardiac disorders : Tachycardia
Respiratory, thoracic and mediastinal disorders : Dyspnoea, Interstitial lung disease
Gastrointestinal disorders : Flatulence, Vomiting, Dry mouth, Stomach discomfort, Dysgeusia
Hepato-biliary disorders : Abnormal hepatic function /liver disorder
Skin and subcutaneous tissue disorders : Pruritus, Hyperhidrosis, Eczema, Erythema,
Muscoloskeletal and connective tissue disorders: arthralgia, pain in extremity
General disorders and administration site conditions : Chest pain, Influenza-like illness
Investigations : Blood creatinine increased, Haemoglobin decreased, Blood uric acid increased,
Hepatic enzyme
increased, Blood phosphokinase increased
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is
important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
In humans, experience with intentional overdose is limited. Dizziness, nausea, somnolence, hypovolaemia, hypotension and electrolyte disturbances associated with cardiac arrhythmias and muscle spasms are some of the signs and symptoms associated with drug overdose. Gastric lavage, emesis or activated charcoal should be employed to reduce absorption. Blood pressure and fluid and electrolyte balance should be monitored and appropriate corrective measures taken. Intravenous fluid and electrolyte replacement may be indicated. Clinically significant hypotension due to drug over dosage calls for active cardiovascular support including frequent monitoring of cardiac and respiratory function, elevation of extremities, and attention to circulating fluid volume and urine output. A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Intravenous Calcium Gluconate may be beneficial in reversing the effects of Calcium channel blockade.
5.0 Pharmacological properties
5.1 Mechanism of action
Efonidipine, a new generation Dihydropyridine (DHP) Calcium channel blocker, inhibits both L-type and T-type Calcium channels.
Telmisartan blocks the vasoconstriction and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor.
5.2 Pharmacodynamic properties
Efonidipine
- Efonidipine exhibits antihypertensive effect through vasodilatation by blocking L-type and T-type Calcium channels.
- Efonidipine has a negative chronotropic effect. Working on sino atrial node cells by inhibiting T-type Calcium channel activation, Efonidipine prolongs the late phase-4 depolarization of the sino atrial node action potential and suppresses an elevated HR. The negative chronotropic effect of Efonidipine decreases heart rate, myocardial oxygen demand and increases coronary blood flow.
- Efonidipine increases coronary blood flow by blocking L & T-type Calcium channels and attenuates myocardial ischemia.
- By reducing synthesis and secretion of aldosterone, Efonidipine prevents hypertrophy and remodeling of cardiac myocytes.
- Efonidipine increases glomerular filtration rate without increasing intra-glomerular pressure and filtration fraction. This prevents hypertension induced renal damage
- Efonidipine prevents Rho-kinase and NF B induced renal parenchymal fibrosis and provides long term renal protection.
- Efonidipine suppresses renin secretion from the juxta glomerular apparatus in the kidneys.
- Efonidipine enhances sodium excretion from the kidneys by suppressing aldosterone synthesis and secretion from the adrenal glands. Aldosterone induced renal parenchymal fibrosis is suppressed by Efonidipine.
- Efonidipine prevents NF B induced hypertrophy and inflammation in the renal vasculature and protects the kidneys.
- Efonidipine protects against endothelial dysfunction due to its anti-oxidant activity and by restoring NO bioavailability.
- Efonidipine has anti-atherogenic activity and protects the blood vessels from atherosclerosis.
- Efonidipine lowers blood pressure in cerebral resistance vessels and prevents hypertension induced brain damage.
Telmisartan
- Telmisartan is an orally active and specific angiotensin II receptor (type AT1) antagonist. Telmisartan displaces angiotensin II with very high affinity from its binding site at the AT1 receptor subtype, which is responsible for the known actions of angiotensin II.
- Telmisartan does not exhibit any partial agonist activity at the AT1 receptor. Telmisartan selectively binds the AT1 receptor. The binding is long-lasting.
- Telmisartan does not show affinity for other receptors, including AT2 and other less characterized AT receptors. The functional role of these receptors is not known, nor is the effect of their possible overstimulation by angiotensin II, whose levels are increased by Telmisartan. Plasma aldosterone levels are decreased by Telmisartan
- Telmisartan does not inhibit human plasma renin or block ion channels. Telmisartan does not inhibit angiotensin converting enzyme (kininase II), the enzyme which also degrades bradykinin. Therefore, it is not expected to potentiate bradykinin-mediated adverse effects.
5.3 Pharmacokinetic properties
Efonidipine
Peak plasma concentration is achieved in about 1.5 to 3.67 hours after administration. The bioavailability of Efonidipine is ~25% and half-life is approximately 4 hours. Efonidipine is primarily metabolized in liver. The important metabolites are N-dephenylated Efonidipine (DPH), deaminated Efonidipine (AL) and N-debenzylated Efonidipine (DBZ). DBZ and DPH exhibit activity as Calcium antagonists. The vasodilating properties of DBZ and DPH are about two-third and one-third respectively than that of parent compound. Biliary route is the main route of excretion. No significant amount of unchanged drug is excreted in urine. In the urine collected for 24 hours after an oral dosing, 1.1% of the dose was excreted as deaminated Efonidipine and 0.5% as a pyridine analogue of deaminated.
Telmisartan
Absorption of Telmisartan is rapid although the amount absorbed varies. The mean absolute bioavailability for Telmisartan is about 50%. When Telmisartan is taken with food, the reduction in the area under the plasma concentration-time curve of Telmisartan varies from approximately 6% (40 mg dose) to approximately 19% (160 mg dose). By 3 hours after administration, plasma concentrations are similar whether Telmisartan is taken fasting or with food. Telmisartan is largely bound to plasma protein (>99.5%), mainly albumin and alpha-1 acid glycoprotein. The mean steady state apparent volume of distribution (Vdss) is approximately 500 L. Telmisartan is metabolised by conjugation to the glucuronide of the parent compound. No pharmacological activity has been shown for the conjugate. Telmisartan is characterised by biexponential decay pharmacokinetics with a terminal elimination half-life of 24 hours.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
No known animal toxicology data
7.0 Description
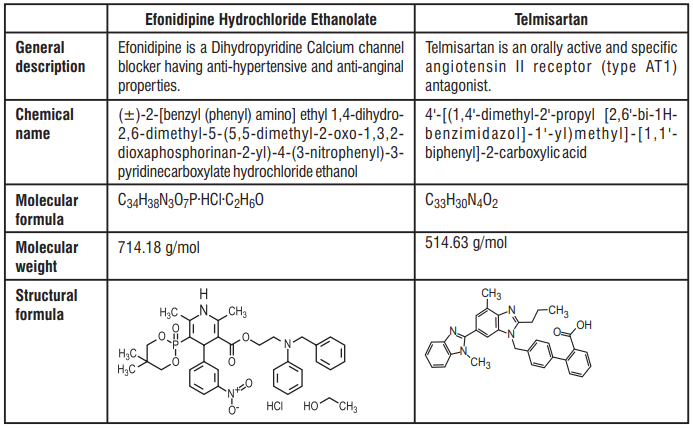
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf life
24 Months
8.3 Packaging information
Efnocar T 20/40 : Alu-Alu blister strip of 10 tablets
Efnocar T 40/40 : Alu-Alu blister strip of 10 tablets.
8.4 Storage and handing Instructions
Store protected from moisture at a temperature not exceeding 30°C
Keep out of reach of children.
9.0 Patient counselling information
Who should not take Efnocar T ?
You should not take Efnocar T tablets if you are allergic (hypersensitive) to the active ingredients (Telmisartan or Efonidipine).
For patients with diabetes, if you are taking Efnocar T you should not take aliskiren
What should I tell my doctor before taking Efnocar T tablets ?
Before you take Efnocar T tablets, tell your doctor if you :
- Have liver problems.
- Have kidney problems.
- Have heart problems.
- Have any other medical conditions.
- Are pregnant or are planning to become pregnant.
- Are breast-feeding or plan to breast-feed. It is not known if Efnocar T passes into your breast milk.
You and your doctor should decide if you will take Efnocar T tablets or breast-feed. You should not do both. Talk with your doctor about the best way to feed your baby if you take Efnocar T tablets. Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
Efnocar T may affect the way other medicines work, and other medicines may affect how Efnocar Tworks. Especially tell your doctor if you take :
- Aliskiren
- Digoxin
- Lithium
- Medicines used to treat pain and arthritis, called non-steroidal anti-inflammatory drugs (NSAIDs), including COX2 inhibitors.
- Ramipril or other medicines that may be used to treat high blood pressure or a heart problem.
- Simvastatin
- Water pills (diuretics)
Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine. How should I take Efnocar T tablets ?
Take Efnocar T tablets exactly as your doctor tells you to take it.
Your doctor will tell you how much Efnocar T to take and when to take it. Your doctor may change your dose if needed.
- Take Efnocar T one time each day at the same time.
- Take Efnocar T tablets with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Take the next dose at your regular time.
- If you take too much Efnocar T, call your doctor or go to the nearest hospital emergency room right away.
What are possible side effects of Efnocar T tablets ?
Efnocar T tablets may cause serious side effects, including :
Injury or death to your unborn baby.
Low blood pressure (hypotension) is most likely to happen if you also :
- Take water pills. (diuretics)
- Are on a low-salt diet.
- Get dialysis treatments
- Have heart problems.
- Get sick with vomiting or diarrhea
If you feel faint or dizzy, lie down and call your doctor right away.
- Kidney problems. Kidney problems may get worse if you already have kidney disease. You may have changes in your kidney test results, and you may need a lower dose of Efnocar T tablets. Call your doctor if you get :
- Swelling in your feet, ankles, or hands
- Unexplained weight gain
Call your doctor right away if you get any of the symptoms listed above.
- Heart problems or heart attack. Heart problems may get worse in people that already have heart disease. This may happen when you start Efnocar T tablets or when there is an increase in your dose of Efnocar T. Get emergency help if you get worse chest pain or chest pain that does not go away.
- High potassium in the blood (hyperkalemia). Your doctor may check your potassium levels as needed.
- Muscle rigidity, tremor and/or abnormal muscle movement
Rare, serious allergic reactions may happen. Tell your doctor right away if you get any of these symptoms :
- Swelling of face, tongue, throat.
- Difficulty breathing.
- Skin rash.
The most common side effects of Efnocar T tablets include :
- Swelling in your hands, ankles, or feet.
- Feeling like your heart is pounding or racing
- Flushing or sudden redness of the face and neck.
- Dizziness
- Back pain.
- Feeling tired or sleepy
- Abdominal pain, nausea, or diarrhea.
- Low blood pressure or a sudden drop in blood pressure with fainting.
These are not all the possible side effects of Efnocar T tablets. Tell your doctor if you have any other side effects.
12.0 Date of issue
03 January 2022
About leaflet
The name of your medicine is EFNOCAR-T 20/40 and 40/40 Tablets. We refer to them as EFNOCAR-T Tablets or EFNOCAR-T throughout this leaflet.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any more questions, please ask your doctor or your pharmacist.
- This medicine has been prescribed for you personally and you should not pass it on to anyone else. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects get serious, or if you notice any side effects that are not listed in the leaflet, please tell your doctor or pharmacist.
In this leaflet:
- What EFNOCAR-T Tablets are and what they are used for
- What you need to know before you take EFNOCAR-T Tablets
- How to take EFNOCAR-T Tablets
- Possible side effects
- How to store EFNOCAR-T Tablets
- Contents of the pack and other information
1. What EFNOCAR-T tablets are and what they are used for
EFNOCAR-T Tablets contain the active substance Efonidipine and Telmisartan. Both of these substances help to control high blood pressure.
- Efonidipine belongs to a group of substances called “calcium channel blockers”. Efonidipine stops calcium from moving into the blood vessel wall which stops the blood vessels from tightening resulting in dilatation of vessel.
- Telmisartan belongs to a group of substances called “angiotensin-II receptor antagonists”. Angiotensin II is produced by the body and makes the blood vessels tighten, thus increasing blood pressure. Telmisartan works by blocking the effect of angiotensin II which helps to reduce the blood pressure.
This means that both of these substances help to stop the blood vessels tightening/constricting. As a result, the blood vessels relax and blood pressure is lowered.
EFNOCAR-T Tablets are used to treat High blood pressure (stage II hypertension)
2. What you need to know before you take EFNOCAR-T Tablets
Do not take EFNOCAR-T Tablets if you:
- Have ever had an allergic reaction to efonidipine or any of the ingredients in the tablet. An allergic reaction may include a rash, itching, difficulty breathing, or swelling of the face, lips, throat, or tongue;
- If you are pregnant
- Have severe liver problems or bile problems such as biliary cirrhosis or cholestasis.
- Have severe low blood pressure (hypotension).
- Have narrowing of the aortic valve (aortic stenosis) or cardiogenic shock (a condition where your heart is unable to supply enough blood to the body).
- Suffer from heart failure after a heart attack.
- Have diabetes or impaired kidney function and you are treated with a blood pressure-lowering medicine containing aliskiren.
If any of the above applies to you, do not take EFNOCAR-T and talk to your doctor.
Take special care with EFNOCAR-T Tablets
You should inform you doctor if you have or have had any of the following conditions:
- Liver disease;
- Recent heart attack;
- Heart failure;
- Severe increase in blood pressure (Hypertensive crisis).
- Kidney disease or kidney transplant.
- Renal artery stenosis (narrowing of the blood vessels to one or both kidneys).
- Raised aldosterone levels (water and salt retention in the body along with imbalance of various blood minerals).
- Low blood pressure (hypotension), likely to occur if you are dehydrated (excessive loss of body water) or have salt deficiency due to diuretic therapy ('water tablets'), low-salt diet, diarrhoea, or vomiting.
- Elevated potassium levels in your blood.
- Diabetes
Use in children and adolescents
EFNOCAR-T is not recommended in infants and children as safety is not established in this group of patients.
For more information, talk to your doctor.
Taking other medicines and EFNOCAR-T
Please tell your doctor or pharmacist if you are taking or have recently taken other medicines, including medicines obtained without a prescription.
EFNOCAR-T may affect or be affected by other medicines, such as:
- Other antihypertensive agent/s (BP lowering drugs) such as ACE-inhibitor (for example enalapril, lisinopril, ramipril)
- Cimetidine (stomach acid reducer)
- Tacrolimus (a drug to suppress immunity)
- Digoxin
EFNOCAR-T may lower your blood pressure even more if you are already taking other medicines to treat your high blood pressure.
If you see another doctor or go into hospital for any reason, tell them that you are taking EFNOCAR-T Tablets.
Taking EFNOCAR-T Tablets with food and drink
You should not drink grapefruit juice or eat grapefruit while taking this medicine. Grapefruit and grapefruit juice can lead to an increase in the blood levels of efonidipine, which can cause an unpredictable increase in its blood pressure-lowering effect.
Pregnancy
The safety of efonidipine in human pregnancy has not been established. You must tell your doctor if you think you are (or might become) pregnant. Your doctor will normally advise you to stop taking EFNOCAR-T Tablets before you become pregnant or as soon as you know you are pregnant and will advise you to take another medicine instead of EFNOCAR-T Tablets. EFNOCAR-T Tablets is not recommended in early pregnancy, and must not be taken when more than 3 months pregnant, as it may cause serious harm to your baby if used after the third month of pregnancy.
Therefore, EFNOCAR-T should not be administered in pregnant women.
Breast-feeding
Tell your doctor if you are breast-feeding or about to start breast-feeding. EFNOCAR-T is not recommended for mothers who are breast-feeding, and your doctor may choose another treatment for you if you wish to breast-feed, especially if your baby is new-born, or was born prematurely.
EFNOCAR-T should not be administered in breastfeeding women.
Driving and using machines
Dizziness may occur while taking antihypertensive agents. Hence, working on aerial platform, working with dangerous machinery and/or driving should be avoided.
3. How to take Efnocar-T tablets
Swallow these tablets with a glass of water at the same time each day. You can take the tablets after meals.
Follow your doctor’s instructions. Check the pharmacy label to see how many tablets to take and how often to take them. If you are still not sure, ask your pharmacist or doctor. The usual doses are described below.
Adults
The usual recommended dose is 1 tablet daily. It may be increased to 2 tablets per day depending upon the blood pressure control or as directed by the Physician.
Efonidipine
Essential hypertension: 20 - 40 mg orally once daily. A dose of up to 80 mg/day has been reported to be safe and effective in clinical trials.
Telmisartan
Essential hypertension: The usually effective dose is 40 mg once daily. In cases where the target blood pressure is not achieved, the dose of Telmisartan can be increased to a maximum of 80 mg once daily.
Children
EFNOCAR-T is not recommended in infants and children as safety is not established in this group of patients.
Elderly
EFNOCAR-T should be started with a low dose. Your doctor will closely monitor your response to any decrease in the dose.
Patients with liver/kidney disease
Your doctor may give you a different dose than normal.
If you take more EFNOCAR-T Tablets than you should
If you (or someone else) swallow a lot of tablets all together, or if you think a child has swallowed any of the tablets, contact your nearest hospital casualty department or your doctor immediately. Take your medication and the packaging with you to the doctor or casualty department. If you have taken an overdose, you may you may appear flushed (your skin will look red), or you may feel dizzy or faint. If blood pressure drop is severe enough shock can occur.
If you forget to take EFNOCAR-T Tablets
If you forget to take a tablet, take one as soon as you remember, unless it is nearly time to take the next one. Never take two doses together. Take the remaining doses at the correct time.
If you stop taking EFNOCAR-T Tablets
Take this medicine for as long as your doctor tells you to, as you may become unwell if you stop.
4. Possible side-effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Efonidipine
The common side effects are hot flushes, palpitations, facial flushing and headache. In addition, elevation in bad cholesterol, liver enzymes may occur.
Other known side effects are as follows. Tell your doctor if you notice or are worried by any of the side effects listed.
Frequency 0.1 to < 5%
- Increased liver enzymes
- Blood Urea Nitrogen rise, blood creatinine rise, proteins in urine
- Decreased haemoglobin, decreased red blood cells
- Rash, itching
- Palpitations, chest pain, decreased blood pressure
- Flushing of the face
- Headache, sluggishness and light-headedness
- Nausea, stomach discomfort, abdominal pain
- Malaise, serum total cholesterol rise, uric acid increased, reduced potassium levels
Frequency Less than 0.1%
- Jaundice
- Reduced platelet count
- Reduced heart rate or increased heart rate
- Hot flushes, sweating
- Drowsiness, numbness, and ringing in ear
- Vomiting, constipation
- Frequent urination, swelling, increased bad cholesterol
Frequency unknown
- Heart failure
- Diarrhea, Gum hypertrophy
Tell your doctor or pharmacist if you notice any other effects not listed.
Telmisartan
You should see your doctor immediately if you experience any of the following symptoms:
Sepsis* (often called "blood poisoning", is a severe infection with whole-body inflammatory response), rapid swelling of the skin and mucosa (angioedema); these side effects are rare but are extremely serious and patients should stop taking the product and see their doctor immediately. If these effects are not treated they could be fatal.
Possible side effects of Telmisartan:
Common side effects (may affect up to 1 in 10 people):
Low blood pressure (hypotension) in users treated for reduction of cardiovascular events.
Uncommon side effects (may affect up to 1 to 100 people):
Urinary tract infections, upper respiratory tract infections (e.g. sore throat, inflamed sinuses, common cold), deficiency in red blood cells (anemia), high potassium levels, difficulty falling asleep, feeling sad (depression), fainting (syncope), feeling of spinning (vertigo), slow heart rate (bradycardia), low blood pressure (hypotension), in users treated for high blood pressure, dizziness on standing up (orthostatic hypotension), shortness of breath, cough, abdominal pain, diarrhea, discomfort in the abdomen, bloating, vomiting, itching, increased sweating, drug rash, back pain, muscle cramps, muscle pain (myalgia), kidney impairment including acute kidney failure, pain in the chest, the feeling of weakness, and increased level of creatinine in the blood.
Rare side effects (may affect up to 1 in 1,000 people):
Sepsis* (often called "blood poisoning", is a severe infection with whole-body inflammatory response which can lead to death), increase in certain white blood cells (eosinophilia), low platelet count (thrombocytopenia), severe allergic reaction (anaphylactic reaction), allergic reaction (e.g. rash, itching, difficulty breathing, wheezing, swelling of the face or low bl ood pressure), low blood sugar levels (in diabetic patients), feeling anxious, somnolence, impaired vision, fast heart beat (tachycardia), upset stomach, dry mouth, taste disturbance (dysgeusia), abnormal liver function(Japanese patients are more likely to experience these side effect), rapid swelling of the skin and mucosa which can also lead to death (angioedema also with fatal outcome), eczema (a skin disorder), redness of skin, hives (urticaria), severe drug rash, joint pain (arthralgia), pain in extremity, tendon pain, flu-like-illness, decreased haemoglobin (a blood protein), increased levels of uric acid, increased hepatic enzymes or creatine phosphokinase in the blood.
Very rare side effects (may affect up to 1 in 10,000 people):
Progressive scarring of lung tissue (interstitial lung disease) **
*The event may have happened by chance or could be related to a mechanism currently not known.
**Cases of progressive scarring of lung tissue have been reported during intake of telmisartan. However, it is not known whether telmisartan was the cause.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Efnocar-T tablets
Do not use the tablets after the end of the expiry month (use-by date) shown on the product packaging.
Store below 25°C. Protected from light & moisture.
Keep this medicine out of the sight and reach of children
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist/doctor how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What EFNOCAR-T Tablets contain
The active substance is Efonidipine and Telmisartan.
Efnocar T 20/40
Each tablet contains: Efonidipine Hydrochloride Ethanolate…20 mg
Telmisartan IP…40 mg
Efnocar T 40/40
Each uncoated bilayered tablet contains: Efonidipine Hydrochloride
Ethanolate 40 mg Telmisartan IP 40 mg
Packaging information
Efnocar T 20/40: An Alu-Alu blister strip of 10 tablets.
Efnocar T 40/40: An Alu-Alu blister strip of 10 tablets.