Elriz XL Syrup
Therapy Area
Respiratory
1.0 Generic Name
Levocetirizine hydrochloride with Ambroxol Hydrochloride Syrup (2.5 mg + 30mg)
2.0 Qualitative and quantitative composition
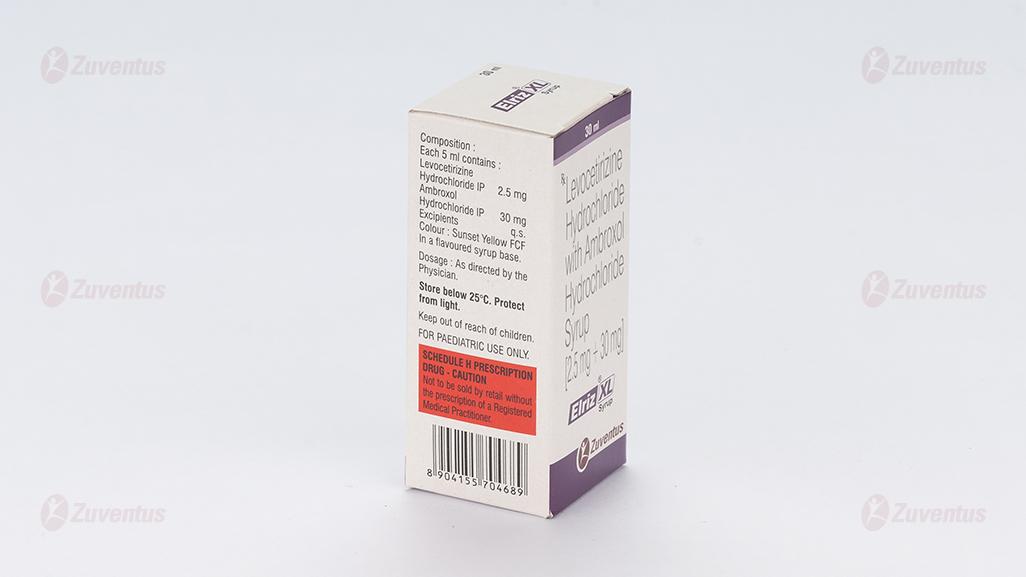
Each 5 ml contains:
Levocetirizine Hydrochloride IP 2.5 mg
Ambroxol Hydrochloride IP 30 mg
Excipients q.s.
Colour: Sunset Yellow FCF
In a flavoured syrup base.
3.0 Dosage form and strength
Syrup for pediatric use only
Levocetirizine 2.5mg and Ambroxol 30mg per 5ml
4.0 Clinical particulars
4.1 Therapeutic Indication
For symptomatic relief of productive cough associated with allergic rhinitis, when both antihistamine and mucolytic agents are desired.
4.2 Posology and method of administration
Children aged 6 to 12 years:
The daily recommended dose is 5 mg (10 ml of syrup).
Children aged 2 to 6 years:
The daily recommended dose is 2.5 mg to be administered in 2 intakes of 1.25 mg (2.5 ml of syrup twice daily).
Even if some clinical data are available in children aged 6 months to 12 years, these data are not sufficient to support the administration of levocetirizine to infants and toddlers aged less than 2 years.
Renal impairment
In paediatric patients suffering from renal impairment, the dose will have to be adjusted on an individual basis taking into account the renal clearance of the patient and his body weight. There are no specific data for children with renal impairment.
Hepatic impairment
No dose adjustment is needed in patients with solely hepatic impairment. In patients with hepatic impairment and renal impairment, adjustment of the dose is recommended (see Renal impairment above).
Method of administration
A measuring cap is included in the package. The appropriate volume of syrup should be measured with the measuring cap. The syrup must be taken orally.
It may be taken with or without food.
4.3 Contraindications
- Hypersensitivity to the active substance, ambroxol or levocetirizine, or to hydroxyzine, to any other piperazine derivatives or to any of the other excipients.
- Patients with end stage renal disease with eGFR below 10 ml/min (requiring dialysis treatment).
- Patients undergoing haemodialysis
4.4 Special warnings and precautions for use
- Caution should be taken in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as levocetirizine may increase the risk of urinary retention.
- Caution should be taken in patients with epilepsy and patients at risk of convulsion as levocetirizine may cause seizure aggravation.
- Response to allergy skin tests are inhibited by antihistamines and a wash-out period (of 3 days) is required before performing them.
- Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
- Pruritus may occur when levocetirizine is stopped even if those symptoms were not present before treatment initiation. The symptoms may resolve spontaneously. In some cases, the symptoms may be intense and may require treatment to be restarted. The symptoms should resolve when the treatment is restarted.
- In clinical trials the occurrence of somnolence, fatigue, and asthenia has been reported in some patients under therapy with Elriz®-XL.
- Concurrent use of Elriz®-XL with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.
- The use of Elriz®-XL should be carefully considered in patients predisposed to peptic ulcers.
- Ciliary dyskinesia: In patients with ciliary dyskinesia the benefit of liquefaction of secretions should be carefully weighed against the risk of congestion of secretions.
4.5 Drugs interactions
- No interaction studies have been performed with levocetirizine (including no studies with CYP3A4 inducers); studies with the racemate compound cetirizine demonstrated that there were no clinically relevant adverse interactions (with anti-pyrine, azithromycin, cimetidine, diazepam, erythromycin, glipizide, ketoconazole and pseudoephedrine). A small decrease in the clearance of cetirizine (16%) was observed in a multiple dose study with theophylline (400 mg once a day); while the disposition of theophylline was not altered by concomitant cetirizine administration.
- In vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes.
- In a multiple dose study of ritonavir (600 mg twice daily) and cetirizine (10 mg daily), the extent of exposure to cetirizine was increased by about 40% while the disposition of ritonavir was slightly altered (-11%) further to concomitant cetirizine administration.
- The extent of absorption of levocetirizine is not reduced with food, although the rate of absorption is decreased.
- In sensitive patients, the concurrent administration of cetirizine or levocetirizine and alcohol or other CNS depressants may cause additional reductions in alertness and impairment of performance.
- After administration of ambroxol concentrations of antibiotics (amoxicillin, cefuroxime, erythromycin) in broncho-pulmonary secretions and sputum is increased.
- Anti-tussives: Concomitant administration of anti-tussives may impair the expectoration of liquefied bronchial mucus due to inhibition of the cough reflex and cause congestion of secretions.
4.6 Use in special populations
Pregnancy, Breast-feeding and Fertility
Not Applicable. This formulation is for paediatric use only.
4.7 Effects on ability to drive and use machines
Not Applicable
4.8 Undesirable effects
Levocetirizine
- Common adverse reactions with (≥1/100 to <1/10): Headache (3.2%), Somnolence (1.4%), dry Mouth (1.6%), and Fatigue (1.2%).
- The incidence of sedating adverse drug reactions such as somnolence, fatigue, and asthenia was altogether more common (8.1%) under compared to placebo (3.1%)
- Uncommon (≥1/1,000 to <1/100): asthenia or abdominal pain
Ambroxol
- Mild upper gastro-intestinal side effects primarily taste disturbances, heartburn, dyspepsia, and occasionally nausea and vomiting have been reported.
- Allergic reactions have occurred rarely, primarily skin rashes.
- There have been extremely rare case reports of severe acute anaphylactic-type reactions but their relationship to Ambroxol is uncertain. Some of these patients have also shown allergic reactions to other substances.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Symptoms:
Levocetirizine
- Symptoms of overdose may include agitation and restlessness may initially occur, followed by drowsiness.
Ambroxol
- There are no severe intoxications known following an overdose of Ambroxol. Manifestations that have been reported include short-term restlessness and diarrhoea. Consistent with the findings of preclinical studies, increased saliva production, nausea, vomiting, and a drop in blood pressure can be observed following extreme overdosing.
Therapeutic Measures: There is no known specific antidote. Should overdose occur, symptomatic or supportive treatment is recommended. Gastric lavage may be considered shortly after ingestion of the drug. Levocetirizine is not effectively removed by haemodialysis.
5.0 Pharmacological properties
5.1 Mechanism of Action
Levocetirizine, the active enantiomer of cetirizine, is a second- generation anti-histamine and a potent and selective antagonist of peripheral H1-receptors. Ambroxol is frequently used as mucolytic and it also induces the synthesis of surfactant in lung alveolar type II cells. By this mechanism, it decreases the work and effort of breathing and can improve respiratory symptoms. It has also been proved to have antioxidant and anti-inflammatory activities
5.2 Pharmacodynamic properties
At half the dose, levocetirizine has comparable activity to cetirizine, both in the skin and in the nose. Levocetirizine shows receptor occupancy of 90% at 4 hours and 57% at 24 hours. The onset of action of levocetirizine 5 mg has been observed at 1-hour post drug intake.
Ambroxol is shown to exert several activities: i) secretolytic activity (i.e., promotes mucus clearance, facilitates expectoration, and eases productive cough); ii) anti-inflammatory and antioxidant activity; and iii) a local anesthetic effect through sodium channel blocking at the level of the cell membrane.
5.3 Pharmacokinetic properties
Levocetirizine exhibited linear pharmacokinetics over the therapeutic dose range in adult healthy subjects.
| Pharmacokinetics parameters | Levocetirizine HCL (5 mg o.d. dose) | Ambroxol HCL |
| Bioavailability | 85% | 79% |
| Cmax | 270 ng/mL and 308 ng/mL | 169.03 ± 23.42 ng/mL |
| Tmax | 0.5 hours | 1.20 ± 0.22 hours |
| Volume of distribution | 0.4 L/kg | 552 L |
| Protein binding | 91 to 92% | 90% |
| Half life | 7.9 ± 1.9 hours | 7.07±1.54 hours |
| Excretion | Renal | Primarily metabolized in the liver |
Pharmacokinetics in special Populations
- Hepatic Insufficiency No dose adjustment is needed in patients with solely hepatic impairment.
- Renal Insufficiency Dosage and frequency of administration of ELRIZ®XL syrup should be reduced.
- Dosage and frequency of administration of ELRIZ®XL syrup should be reduced in patients with mild, moderate or severe renal impairment
- In patients with moderate to severe renal /hepatic impairment, a slower elimination rate may lead to the accumulation of ambroxol and/or ambroxol-metabolites formed in the liver.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential.
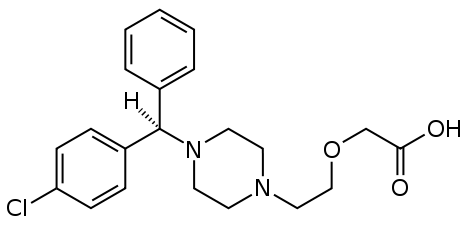
7.0 Description
ELRIZ®-XL syrup is a combination of a Levocetirizine Hydrochloride (2.5mg) and Ambroxol Hydrochloride (30mg).
Levocetirizine hydrochloride is an orally active H1-receptor antagonist.
Molecular Formula: C21H25ClN2O3•HCl
Chemical name: (R)-[2-[4-[(4-chlorophenyl) phenylmethyl]-1-piperazinyl] ethoxy] acetic acid hydrochloride.
Molecular weight: 461.82
Molecular Structure:
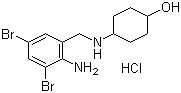
Ambroxol is a metabolite of bromhexine and is widely used as a mucolytic.
Molecular Formula: C13H18Br2N2O.HCl
Chemical name: trans-4-((2-amino-3,5-dibromobenzyl) amino) cyclohexanol hydrochloride
Molecular Weight: 414.56 g/mol
Molecular Structure:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on the pack
8.3 Packaging information
A bottle of 30 mL For pediatric use only.
8.4 Storage and handing instructions
Store in cool and dark place.
Keep out of reach of children.
9.0 Patient Counselling Information
- Somnolence
- Concomitant Use of Alcohol and other Central Nervous System Depressants Instruct patients to avoid concurrent use of levocetirizine hydrochloride with alcohol or other central nervous system depressants because additional reduction in mental alertness may occur.
- Dosing Advise patients to not ingest more than the recommended dose of ELRIZ®-XL syrup because of the increased risk of somnolence at higher doses.
- Do not take this medicine, if you are allergic to Levocetirizine and/or Ambroxol Signs of an allergic reaction include a rash, itching or shortness of breath.
- Advice patients to talk to your doctor or pharmacist, if you get any side effects. This includes any possible side effects not listed in this leaflet.
- Advice patients if they have any further questions, ask doctor or pharmacist.
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1.What ELRIZ®XL Syrup is and what it is used for
2.What you need to know before you take ELRIZ®XL syrup
3.How to take ELRIZ®XL syrup
4.Possible side effects
5.How to store ELRIZ®XL syrup
6.Contents of the pack and other information
1. What ELRIZ®XL syrup is and what it is used for
ELRIZ®XL syrup is a fixed dose combination of a Levocetirizine Hydrochloride (2.5mg) and Ambroxol Hydrochloride (30mg).
Levocetirizine, the active enantiomer of cetirizine, is a second-generation anti-histamine and a potent and selective antagonist of peripheral H1-receptors.
Ambroxol is frequently used as mucolytic and it also induces the synthesis of surfactant in lung alveolar type II cells. By this mechanism, it decreases the work and effort of breathing and can improve respiratory symptoms. It has also been proved to have antioxidant and anti-inflammatory activities Levocetirizine dihydrochloride is the active ingredient of ELRIZ®XL syrup.
ELRIZ®XL syrup is used for symptomatic relief of productive cough associated with allergic rhinitis, when both antihistamine and mucolytic agents are desired.
2. What you need to know before you take ELRIZ®XL Syrup
Do not take ELRIZ®XL Syrup:
- If you are allergic to ELRIZ®XL syrup or any of its ingredients.
- If you have a severe impairment of kidney function (severe renal failure with creatinine clearance below 10 ml/min).
- If you have a severe kidney disease requiring dialysis.
Warnings and precautions
Talk to your doctor before taking ELRIZ®XL:
- If you are likely to be unable to empty your bladder (with conditions such as spinal cord injury or enlarged prostate), please ask your doctor for advice.
- If you suffer from epilepsy or are at risk of convulsions, please ask your doctor for advice as use of ELRIZ®XL syrup may cause seizure aggravation.
- If you are scheduled for allergy testing, ask your doctor if you should stop taking ELRIZ®XL syrup for several days before testing. This medicine may affect your allergy test results.
Tell your doctor immediately if during treatment you suffer from
Hypersensitivity reactions (swelling of the mouth, tongue, face and/or throat, breathing or swallowing difficulties, chest tightness or wheezing, hives, sudden fall in blood pressure)
At the first signs of a hypersensitivity reaction, stop taking ELRIZ®XL syrup and tell your doctor.
Other medicines and ELRIZ®XL Syrup
Tell your doctor if you are taking or have taken the following medications.
- Ritonavir
- Antibiotics (Amoxicillin, Cefuroxime, Erythromycin)
- Antitussives
ELRIZ®XL Syrup with food, drink and alcohol
Take caution during the concurrent administration of ELRIZ®XL syrup and alcohol or other agents.
ELRIZ®XL syrup can be taken with or without food.
Pregnancy, breast-feeding and fertility
This formulation is for paediatric use only.
Driving and using machines
This formulation is for paediatric use only.
3. How to take ELRIZ®XL Syrup
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure.
The recommended dose:
Children aged 6 to 12 years: 10 mL of syrup once daily
Children aged 2 to 6 years: 2.5 ml of syrup twice daily
Special dosage instructions for specific populations:
Renal and hepatic impairment
Patients with impaired kidney function may be given a lower dose according to the severity of their kidney disease, and in children the dose will also be chosen on the basis of body weight; the dose will be determined by your doctor.
Patients who have severe impairment of kidney function must not take ELRIZ®XL Syrup.
Patients who only have impaired liver function should take the usual prescribed dose.
Patients who have both impaired liver and kidney function may be given a lower dose depending on the severity of the kidney disease, and in children the dose will also be chosen on the basis of body weight; the dose will be determined by your doctor.
If you take more ELRIZ®XL Syrup than you should
If you take more ELRIZ®XL syrup than you should, somnolence can occur in adults. Children may initially show excitation and restlessness followed by somnolence.
Tell your doctor or emergency department in hospital immediately.
If you forget to take ELRIZ®XL Syrup
If you forget to take ELRIZ®XL syrup, or if you take a dose lower than that prescribed by your doctor, do not take a double dose to make up for a forgotten dose. Take your next dose at your normal time.
If you stop taking ELRIZ®XL Syrup
Stopping treatment should have no negative effects. However, rarely pruritus (intense itching) may occur if you stop taking ELRIZ®XL syrup, even if those symptoms were not present before treatment initiation.
The symptoms may resolve spontaneously. In some cases, the symptoms may be intense and may require treatment to be restarted. The symptoms should resolve when the treatment is restarted.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Tell your doctor, nurse or pharmacist immediately if you notice any of these side effects during your treatment with ELRIZ®XL Syrup:
Levocetirizine
- Common adverse reactions with (≥1/100 to <1/10): Headache (3.2%), Somnolence (1.4%), dry Mouth (1.6%), and Fatigue (1.2%).
- The incidence of sedating adverse drug reactions such as somnolence, fatigue, and asthenia was altogether more common (8.1%) under compared to placebo (3.1%)
- Uncommon (≥1/1,000 to <1/100): asthenia or abdominal pain
Ambroxol
- Mild upper gastro-intestinal side effects primarily Taste disturbances, heartburn, dyspepsia, and occasionally nausea and vomiting have been reported.
- Allergic reactions have occurred rarely, primarily skin rashes.
- There have been extremely rare case reports of severe acute anaphylactic-type reactions but their relationship to Ambroxol is uncertain. Some of these patients have also shown allergic reactions to other substances.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store ELRIZ®XL Syrup
Store in cool and dark place.
Keep out of reach of children.
6. Contents of the pack and other information
What ELRIZ®XL Syrup contains
Each 5 ml contains:
Levocetirizine Hydrochloride IP 2.5 mg
Ambroxol Hydrochloride IP 30 mg
Excipients q.s.
Colour: Sunset Yellow FCF
In a flavoured syrup base.
Pack size: A bottle of 30 mL