Emigo MD Tablets
Therapy Area
Gastrointestinal
1.0 Generic name
Ondansetron IP
2.0 Qualitative and quantitative composition
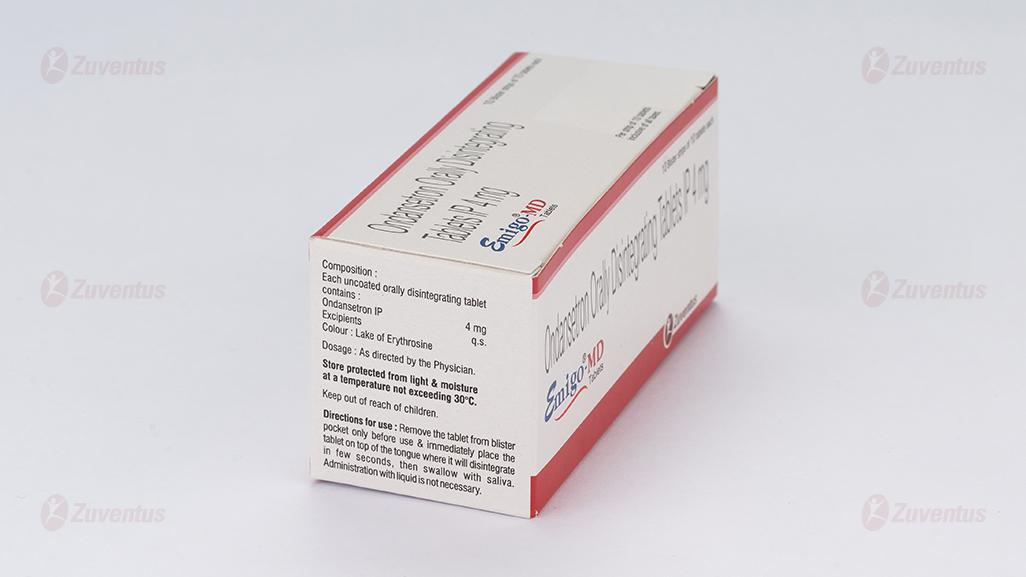
Each uncoated orally disintegrating tablet contains:
Ondansetron IP 4 mg.
Excipients q.s.
Colour: Lake Erythrosine.
3.0 Dosage form and strength
Orally disintegrating (Mouth Dissolving) tablet 4 mg.
4.0 Clinical particulars
4.1. Therapeutic indication
Adults:
Emigo®-MD Tablets is indicated in adults for the management of nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy. Emigo®-MD Tablets is indicated for the prevention of post-operative nausea and vomiting (PONV).
Paediatric Population:
Emigo®-MD Tablets is indicated in children aged ≥6 months for the management of chemotherapy-induced nausea and vomiting (CINV).
No studies have been conducted on the use of orally administered ondansetron in the prevention and treatment of PONV in children aged ≥1 month, administration by IV injection is recommended for this purpose.
4.2. Posology and method of administration
Place the mouth dissolving tablet on top of the tongue, where it will disperse within seconds, then swallow.
Chemotherapy and radiotherapy induced nausea and vomiting
Adults:
The emetogenic potential of cancer treatment varies according to the doses and combinations of chemotherapy and radiotherapy regimens used. The selection of dose regimen should be determined by the severity of the emetogenic challenge.
Emetogenic chemotherapy and radiotherapy:
Ondansetron can be given either by rectal, oral (as a mouth dissolving tablet), intravenous, or intramuscular administration.
For oral administration: 8 mg taken 1 to 2 hours before chemotherapy or radiation treatment, followed by 8 mg every 12 hours for a maximum of 5 days to protect against delayed or prolonged emesis.
For highly emetogenic chemotherapy:
A single dose of up to 24 mg Ondansetron taken with 12 mg oral dexamethasone sodium phosphate, 1 to 2 hours before chemotherapy, may be used. To protect against delayed or prolonged emesis after the first 24 hours, treatment with Ondansetron may be continued for up to 5 days after a course of treatment. The recommended dose is 8 mg to be taken twice daily.
Paediatric population:
CINV in children aged ≥ 6 months and adolescents. The dose for CINV can be calculated based on body surface area (BSA) or weight – see below. In paediatric clinical studies, ondansetron was given by IV infusion diluted in 25 to 50 mL of saline or other compatible infusion fluid and infused over not less than 15 minutes. Weight-based dosing results in higher total daily doses compared to BSA-based dosing.
There are no data from controlled clinical trials on the use of Ondansetron in the prevention of delayed or prolonged CINV. There are no data from controlled clinical trials on the use of Ondansetron for radiotherapy-induced nausea and vomiting in children.
Dosing by BSA:
Ondansetron should be administered immediately before chemotherapy as a single intravenous dose of 5 mg/m2. The single intravenous dose must not exceed 8 mg. Oral dosing can commence 12 hours later and may be continued for up to 5 days (Table 1).
The total dose over 24 hours (given as divided doses) must not exceed adult dose of 32 mg.
Table 1: BSA-based dosing for Chemotherapy - Children aged ≥6 months
and adolescents.
| BSA | Day 1(a,b) | Days 2-6 (b) |
| <0.6m2 | 5mg/m2IV plus 2mg syrup after 12 hours | 2mg syrup every 12 hours |
| ≥0.6m2to ≤1.2m2 | 5mg/m2 IV plus 4 mg syrup or tablet after 12 hours | 4mg syrup or tablet every 12 hours |
| >1.2m2 | 5mg/m2 or 8mg IV plus 8mg syrup or tablet after 12 hours | 8mg syrup or tablet every 12 hours |
a. The intravenous dose must not exceed 8mg.
b. The total dose over 24 hours (given as divided doses) must not exceed adult dose of 32mg.
Dosing by bodyweight:
Weight-based dosing results in higher total daily doses compared to BSA-based dosing.
Ondansetron should be administered immediately before chemotherapy as a single intravenous dose of 0.15mg/kg. The single intravenous dose must not exceed 8 mg. Two further intravenous doses may be given in 4-hourly intervals.
Oral dosing can commence 12 hours later and may be continued for up to 5 days (Table 2).
The total dose over 24 hours (given as divided doses) must not exceed adult dose of 32mg.
Table 2: Weight-based dosing for Chemotherapy - Children aged ≥6 months and adolescents.
| Weight | Day 1 (a,b) | Days 2-6 (b) |
| ≤ 10kg | Up to 3 doses of 0.15mg/kg IV every 4 hours | 2 mg syrup every 12 hours |
| >10kg | Up to 3 doses of 0.15mg/kg IV every 4 hours | 4 mg syrup or tablet every 12 hours |
a. The intravenous dose must not exceed 8mg.
b. The total dose over 24 hours (given as divided doses) must not exceed adult dose of 32mg.
Elderly:
No alteration of oral dose or frequency of administration is required.
Post-operative nausea and vomiting (PONV)
Adults:
For the prevention of PONV: Ondansetron may be administered either orally (as an mouth dissolving tablet) or by intravenous or intramuscular injection. For oral administration: 16 mg taken one hour prior to anaesthesia. For the treatment of established PONV: Intravenous or intramuscular administration is recommended.
Paediatric population:
PONV in children aged ≥ 1 month and adolescents
Oral formulation:
No studies have been conducted on the use of orally administered ondansetron in the prevention or treatment of post-operative nausea and vomiting; slow IV injection (not less than 30 seconds) is recommended for this purpose.
Injection:
For prevention of PONV in paediatric patients having surgery performed under general anaesthesia, a single dose of ondansetron may be administered by slow intravenous injection (not less than 30 seconds) at a dose of 0.1mg/kg up to a maximum of 4mg either prior to, at or after induction of anaesthesia. For the treatment of PONV after surgery in paediatric patients having surgery performed under general anaesthesia, a single dose of ondansetron may be administered by slow intravenous injection (not less than 30 seconds) at a dose of 0.1mg/kg up to a maximum of 4mg.
There are no data on the use of Ondansetron in the treatment of PONV in children below 2 years of age.
Elderly:
There is limited experience in the use of Ondansetron in the prevention and treatment of PONV in the elderly, however Ondansetron is well tolerated in patients over 65 years receiving chemotherapy.
For both indications
Patients with renal impairment:
No alteration of daily dosage or frequency of dosing, or route of administration are required.
Patients with hepatic impairment:
Clearance of Ondansetron is significantly reduced and serum half-life significantly prolonged in subjects with moderate or severe impairment of hepatic function. In such patients, a total daily dose of 8mg should not be exceeded.
Patients with poor sparteine/debrisoquine metabolism: The elimination half-life of ondansetron is not altered in subjects classified as poor metabolisers of sparteine and debrisoquine. Consequently, in such patients, repeat dosing will give drug exposure levels no different from those of the general population. No alteration of daily dosage or frequency of dosing is required.
4.3. Contraindications
Concomitant use with apomorphine.
Hypersensitivity to the active substance or to any of the excipients.
4.4. Special warnings and precautions for use
Hypersensitivity reactions have been reported in patients who have exhibited hypersensitivity to other selective 5HT3 receptor antagonists. Respiratory events should be treated symptomatically and clinicians should pay particular attention to them as precursors of hypersensitivity reactions.
Ondansetron prolongs the QT interval in a dose-dependent manner. In addition, post-marketing cases of Torsade de Pointes have been reported in patients using ondansetron. Avoid ondansetron in patients with congenital long QT syndrome. Ondansetron should be administered with caution to patients who have or may develop prolongation of QTc, including patients with electrolyte abnormalities, congestive heart failure, bradyarrhythmias or patients taking other medicinal products that lead to QT prolongation or electrolyte abnormalities.
Hypokalemia and hypomagnesaemia should be corrected prior to ondansetron administration.
There have been post-marketing reports describing patients with serotonin syndrome (including altered mental status, autonomic instability and neuromuscular abnormalities) following the concomitant use of ondansetron and other serotonergic drugs (including selective serotonin reuptake inhibitors (SSRI) and serotonin noradrenaline reuptake inhibitors (SNRIs)). If concomitant treatment with ondansetron and other serotonergic drugs is clinically warranted, appropriate observation of the patient is advised.
As ondansetron is known to increase large bowel transit time, patients with signs of subacute intestinal obstruction should be monitored following administration. In patients with adenotonsillar surgery, prevention of nausea and vomiting with ondansetron may mask occult bleeding. Therefore, such patients should be followed carefully after ondansetron.
Paediatric population
Paediatric patients receiving ondansetron with hepatotoxic chemotherapeutic agents should be monitored closely for impaired hepatic function.
CINV
When calculating the dose on an mg/kg basis and administering three doses at 4-hour intervals, the total daily dose will be higher than if one single dose of 5 mg/m2 followed by an oral dose is given. The comparative efficacy of these two different dosing regimens has not been investigated in clinical trials. Cross-trial comparison indicates similar efficacy for both regimens.
4.5. Interaction with other medicinal products and other forms of interaction
There is no evidence that ondansetron either induces or inhibits the metabolism of other drugs commonly co-administered with it. Specific studies have shown that there are no interactions when ondansetron is administered with alcohol, temazepam, furosemide, alfentanil, tramadol, morphine, lidocaine, thiopental or propofol. Ondansetron is metabolised by multiple hepatic cytochrome P-450 enzymes: CYP3A4, CYP2D6 and CYP1A2. Due to the multiplicity of metabolic enzymes capable of metabolising ondansetron, enzyme inhibition or reduced activity of one enzyme (e.g. CYP2D6 genetic deficiency) is normally compensated by other enzymes and should result in little or no significant change in overall ondansetron clearance or dose requirement.
Caution should be exercised when ondansetron is co-administered with drugs that prolong the QT interval and/or cause electrolyte abnormalities. Use of ondansetron with QT prolonging drugs may result in additional QT prolongation. Concomitant use of ondansetron with cardiotoxic drugs (e.g. anthracyclines (such as doxorubicin, daunorubicin) or trastuzumab), antibiotics (such as erythromycin), antifungals (such as ketoconazole), antiarrhythmics (such as amiodarone) and beta blockers (such as atenolol or timolol) may increase the risk of arrhythmias.
Serotonergic Drugs (e.g. SSRIs and SNRIs): There have been post-marketing reports describing patients with serotonin syndrome (including altered mental status, autonomic instability and neuromuscular abnormalities) following the concomitant use of ondansetron and other serotonergic drugs (including SSRIs and SNRIs). Apomorphine: Based on reports of profound hypotension and loss of consciousness when ondansetron was administered with apomorphine hydrochloride, concomitant use with apomorphine is contraindicated.
Phenytoin, Carbamazepine and Rifampicin: In patients treated with potent inducers of CYP3A4 (i.e. phenytoin, carbamazepine, and rifampicin), the oral clearance of ondansetron was increased and ondansetron blood concentrations were decreased. Tramadol: Data from small studies indicate that ondansetron may reduce the analgesic effect of tramadol.
4.6. Fertility, pregnancy and lactation
Women of childbearing potential
Women of childbearing potential should consider the use of contraception.
Pregnancy
Based on human experience from epidemiological studies, ondansetron is suspected to cause orofacial malformations, when administered during the first trimester of pregnancy.
In one cohort study including 1.8 million pregnancies, first trimester ondansetron use was associated with an increased risk of oral clefts (3 additional cases per 10,000 women treated; adjusted relative risk, 1.24, (95% CI 1.03-1.48)). The available epidemiological studies on cardiac malformations show conflicting results. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity.
Ondansetron should not be used during the first trimester of pregnancy.
Breast-feeding
Tests have shown that ondansetron passes into the milk of lactating animals. It is, therefore, recommended that mothers receiving ondansetron should not breast-feed their babies.
Fertility
There is no information on the effects of ondansetron on human fertility.
4.7. Effects on ability to drive and use machines
In psychomotor testing, ondansetron does not impair performance nor cause sedation. No detrimental effects on such activities are predicted from the pharmacology of ondansetron.
4.8. Undesirable effects
Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) and very rare (<1/10,000) including isolated reports. Very common, common and uncommon events were generally determined from clinical trial data. The incidence in placebo was taken into account. Rare and very rare events were generally determined from post-marketing spontaneous data. The following frequencies are estimated at the standard recommended doses of ondansetron according to indication and formulation. The adverse event profiles in children and adolescents were comparable to that seen in adults.
The following frequencies are estimated at the standard recommended doses of ondansetron. The adverse event profiles in children and adolescents were comparable to that seen in adults.


1 Observed without definitive evidence of persistent clinical sequelae.
2 The majority of the blindness cases reported resolved within 20 minutes. Most patients had received chemotherapeutic agents, which included cisplatin. Some cases of transient blindness were reported as cortical in origin.
3 These events were observed commonly in patients receiving chemotherapy with cisplatin.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9. Overdose
Symptoms
There is limited experience of ondansetron overdose. In the majority of cases, symptoms were similar to those already reported in patients receiving recommended doses. Manifestations that have been reported include visual disturbances, severe constipation, hypotension, and a vasovagal episode with transient second-degree AV block.
Ondansetron prolongs QT interval in a dose-dependent manner. ECG monitoring is recommended in cases of overdose. Cases consistent with serotonin syndrome have been reported in young children following oral overdose.
Management
There is no specific antidote for ondansetron, therefore in cases of suspected overdose, symptomatic and supportive therapy should be given as appropriate. Further management should be as clinically indicated or as recommended by the national poisons centre, where available. The use of ipecacuanha to treat overdose with ondansetron is not recommended, as patients are unlikely to respond due to the anti-emetic action of ondansetron itself.
Paediatric population
Paediatric cases consistent with serotonin syndrome have been reported after inadvertent oral overdoses of ondansetron (exceeded estimated ingestion of 4 mg/kg) in infants and children aged 12 months to 2 years.
5.0 Pharmacological properties
1.Pharmacodynamic properties
Mechanism of action
Ondansetron is a potent, highly selective 5HT3 receptor-antagonist. Its precise mode of action in the control of nausea and vomiting is not known. Chemotherapeutic agents and radiotherapy may cause release of 5HT in the small intestine initiating a vomiting reflex by activating vagal afferents via 5HT3 receptors. Ondansetron blocks the initiation of this reflex. Activation of vagal afferents may also cause a release of 5HT in the area postrema, located on the floor of the fourth ventricle, and this may also promote emesis through a central mechanism. Thus, the effect of ondansetron in the management of the nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy is probably due to antagonism of 5HT3 receptors on neurons located both in the peripheral and central nervous system.
The mechanisms of action in post-operative nausea and vomiting are not known but there may be common pathways with cytotoxic induced nausea and vomiting.
Ondansetron does not alter plasma prolactin concentrations. The role of ondansetron in opiate-induced emesis is not yet established
QT prolongation
The effect of ondansetron on the QTc interval was evaluated in a double blind, randomised, placebo and positive (moxifloxacin) controlled, crossover study in 58 healthy adult men and women.
Ondansetron doses included 8 mg and 32 mg infused intravenously over 15 minutes. At the highest tested dose of 32 mg, the maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction was 19.6 (21.5) msec. At the lower tested dose of 8 mg, the maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction was 5.8 (7.8) msec. In this study, there were no QTcF measurements greater than 480 msec and no QTcF prolongation was greater than 60 msec.
Paediatric population
CINV
The efficacy of ondansetron in the control of emesis and nausea induced by cancer chemotherapy was assessed in a double-blind randomised trial in 415 patients aged 1 to 18 years (S3AB3006). On the days of chemotherapy, patients received either ondansetron 5 mg/m2 intravenous + ondansetron 4 mg orally after 8-12 hours or ondansetron 0.45 mg/kg intravenous + placebo orally after 8-12 hours. Post-chemotherapy both groups received 4 mg ondansetron syrup twice daily for 3 days. Complete control of emesis on worst day of chemotherapy was 49% (5 mg/m2 intravenous + ondansetron 4 mg orally) and 41% (0.45 mg/kg intravenous + placebo orally). Post-chemotherapy both groups received 4 mg ondansetron syrup twice daily for 3 days. There was no difference in the overall incidence or nature of adverse events between the two treatment groups.
A double-blind randomised placebo-controlled trial (S3AB4003) in 438 patients aged 1 to 17 years demonstrated complete control of emesis on worst day of chemotherapy in:
- 73% of patients when ondansetron was administered intravenously at a dose of 5 mg/m2 intravenous together with 2-4 mg dexamethasone orally.
- 71% of patients when ondansetron was administered as syrup at a dose of 8 mg + 2-4 mg dexamethasone orally on the days of chemotherapy.
Post-chemotherapy both groups received 4 mg ondansetron syrup twice daily for 2 days. There was no difference in the overall incidence or nature of adverse events between the two treatment groups.
The efficacy of ondansetron in 75 children aged 6 to 48 months was investigated in an open-label, non-comparative, single-arm study (S3A40320). All children received three 0.15 mg/kg doses of intravenous ondansetron, administered 30 minutes before the start of chemotherapy and then at four and eight hours after the first dose. Complete control of emesis was achieved in 56% of patients.
Another open label, non-comparative, single-arm study (S3A239) investigated the efficacy of one intravenous dose of 0.15 mg/kg ondansetron followed by two oral ondansetron doses of 4 mg for children aged < 12 years and 8 mg for children aged ≥ 12 years (total no. of children n=28). Complete control of emesis was achieved in 42% of patients.
PONV
The efficacy of a single dose of ondansetron in the prevention of post-operative nausea and vomiting was investigated in a randomised, double-blind, placebo-controlled study in 670 children aged 1 to 24 months (post-conceptual age ≥ 44 weeks, weight ≥ 3 kg). Included subjects were scheduled to undergo elective surgery under general anaesthesia and had an ASA status ≤ III. A single dose of ondansetron 0.1 mg/kg was administered within five minutes following induction of anaesthesia. The proportion of subjects who experienced at least one emetic episode during the 24-hour assessment period (ITT) was greater for patients on placebo than those receiving ondansetron (28% vs. 11%, p < 0.0001).
Four double-blind, placebo-controlled studies have been performed in 1469 male and female patients (2 to 12 years of age) undergoing general anaesthesia. Patients were randomised to either single intravenous doses of ondansetron (0.l mg/kg for paediatric patients weighing 40 kg or less, 4 mg for paediatric patients weighing more than 40 kg; number of patients = 735) or placebo (number of patients = 734). Study drug was administered over at least 30 seconds, immediately prior to or following anaesthesia induction. Ondansetron was significantly more effective than placebo in preventing nausea and vomiting. The results of these studies are summarised in Table 3.
Table 3. Prevention and treatment of PONV in Paediatric Patients – Treatment response over 24 hours
| Study | Endpoint | Ondansetron % | Placebo % | p value |
| S3A380 | CR | 68 | 39 | ≤0.001 |
| S3GT09 | CR | 61 | 35 | ≤0.001 |
| S3A381 | CR | 53 | 17 | ≤0.001 |
| S3GT11 | No nausea | 64 | 51 | 0.004 |
| S3GT11 | No emesis | 60 | 47 | 0.004 |
CR = no emetic episodes, rescue or withdrawal
2.Pharmacokinetic properties
Absorption
Following oral administration of ondansetron, absorption is rapid with maximum peak plasma concentrations of about 30ng/mL being attained and achieved in approximately 1.5 hours after an 8mg dose.
Distribution
The syrup and tablet formulations are bioequivalent and have an absolute oral bioavailability of 60%.
Elimination
The disposition of ondansetron following oral, intravenous and intramuscular dosing is similar with a terminal elimination half-life of approximately 3 hours and a steady- state volume of distribution of about 140L. Ondansetron is not highly protein bound (70-76%) and is cleared from the systemic circulation predominantly by hepatic metabolism through multiple enzymatic pathways. Less than 5% of the absorbed dose is excreted unchanged in the urine. The absence of the enzyme CYP2D6 (the debrisoquine polymorphism) has no effect on the pharmacokinetics of ondansetron. The pharmacokinetic properties of ondansetron are unchanged on repeat dosing.
Special Patient Populations
Gender Gender differences were shown in the disposition of ondansetron, with females having a greater rate and extent of absorption following an oral dose and reduced systemic clearance and volume of distribution (adjusted for weight). Children and Adolescents (aged 1 month to 17 years) In paediatric patients aged 1 to 4 months (n=19) undergoing surgery, weight normalised clearance was approximately 30% slower than in patients aged 5 to 24 months (n=22) but comparable to the patients aged 3 to 12 years. The half-life in the patient population aged 1 to 4 months was reported to average 6.7 hours compared to 2.9 hours for patients in the 5 to 24 month and 3 to 12 year age range. The differences in pharmacokinetic parameters in the 1 to 4 month patient population can be explained in part by the higher percentage of total body water in neonates and infants and a higher volume of distribution for water soluble drugs like ondansetron. In paediatric patients aged 3 to 12 years undergoing elective surgery with general anaesthesia, the absolute values for both the clearance and volume of distribution of ondansetron were reduced in comparison to values with adult patients. Both parameters increased in a linear fashion with weight and by 12 years of age, the values were approaching those of young adults. When clearance and volume of distribution values were normalised by body weight, the values for these parameters were similar between the different age group populations. Use of weight-based dosing compensates for age-related changes and is effective in normalising systemic exposure in paediatric patients. Population pharmacokinetic analysis was performed on 428 subjects (cancer patients, surgery patients and healthy volunteers) aged 1 month to 44 years following intravenous administration of ondansetron. Based on this analysis, systemic exposure (AUC) of ondansetron following oral or IV dosing in children and adolescents was comparable to adults, with the exception of infants aged 1 to 4 months. Volume was related to age and was lower in adults than in infants and children. Clearance was related to weight but not to age with the exception of infants aged 1 to 4 months. It is difficult to conclude whether there was an additional reduction in clearance related to age in infants 1 to 4 months or simply inherent variability due to the low number of subjects studied in this age group. Since patients less than 6 months of age will only receive a single dose in PONV a decreased clearance is not likely to be clinically relevant.
Elderly
Early Phase I studies in healthy elderly volunteers showed a slight age-related decrease in clearance, and an increase in half-life of ondansetron. However, wide inter-subject variability resulted in considerable overlap in pharmacokinetic parameters between young (< 65 years of age) and elderly subjects (≥ 65 years of age) and there were no overall differences in safety or efficacy observed between young and elderly cancer patients enrolled in CINV clinical trials to support a different dosing recommendation for the elderly. Based on more recent ondansetron plasma concentrations and exposure-response modelling, a greater effect on QTcF is predicted in patients ≥75 years of age compared to young adults. Specific dosing information is provided for patients over 65 years of age and over 75 years of age for intravenous dosing.
Renal impairment
In patients with renal impairment (creatinine clearance 15-60mL/min), systemic clearance and volume of distribution are reduced, resulting in a slight, but clinically insignificant increase in elimination half-life (5.4 hours). A study in patients with severe renal impairment who required regular haemodialysis (studied between dialyses) showed ondansetron's pharmacokinetics to be essentially unchanged.
Hepatic impairment
In patients with severe hepatic impairment, systemic clearance is markedly reduced with prolonged elimination half-lives (15-32 hours) and an oral bioavailability approaching 100% because of reduced pre-systemic metabolism.
6.0 Nonclinical properties
No additional data of relevance.
7.0 Description
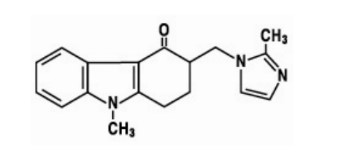
The active ingredient in EMIGO MD® tablets is ondansetron, the racemic form of ondansetron, and a selective blocking agent of the serotonin 5-HT3 receptor type.
8.0 Pharmaceutical particulars
1.Incompatibilities
Not applicable.
2.Shelf-life
Refer on pack
3.Packaging information
10 Blister strips of 10 tablets each
4.Storage and handing instructions
Store protected from light & moisture
at a temperature not exceeding 30°C.
Keep out of reach of children.
9.0 Patient Counselling Information
Directions for use:
Remove the tablet from blister pocket only before use & immediately place the tablet on top of the tongue where it will disintegrate in few seconds, then swallow with saliva.
Administration with liquid is not necessary.
QT Prolongation
Inform patients that EMIGO® may cause serious cardiac arrhythmias such as QT prolongation. Instruct patients to tell their healthcare provider right away if they perceive a change in their heart rate, if they feel lightheaded, or if they have a syncopal episode.
Hypersensitivity Reactions
Inform patients that EMIGO® may cause hypersensitivity reactions, some as severe as anaphylaxis and bronchospasm. Instruct patients to immediately report any signs and symptoms of hypersensitivity reactions, including fever, chills, rash, or breathing problems to their healthcare provider.
Masking of Progressive Ileus and Gastric Distension
Inform patients following abdominal surgery or those with chemotherapy-induced nausea and vomiting that EMIGO® may mask signs and symptoms of bowel obstruction. Instruct patients to immediately report any signs or symptoms consistent with a potential bowel obstruction to their healthcare provider.
Drug Interactions
- Instruct the patient to report the use of all medications, especially apomorphine, to their healthcare provider. Concomitant use of apomorphine and EMIGO® may cause a significant drop in blood pressure and loss of consciousness.
- Advise patients of the possibility of serotonin syndrome with concomitant use of EMIGO® and another serotonergic agent such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur: changes in mental status, autonomic instability, neuromuscular symptoms with or without gastrointestinal symptoms.
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Emigo®-MD Tablet is and what it is used for
2. What you need to know before you take Emigo®-MD Tablet
3. How to take Emigo®-MD Tablet
4. Possible side effects
5. How to store Emigo®-MD Tablet
6. Contents of the pack and other information
1. What Emigo®-MD Tablet is and what it is used for
Emigo®-MD tablets are Mouth Dissolving (orally disintegrating) Tablets and contain a medicine called ondansetron. This belongs to a group of medicines called anti-emetics. Ondansetron works by blocking signals in the brain that cause nausea and vomiting. Emigo®-MD Tablet are a special type of tablet that dissolves very quickly when put on top of the tongue.
Ondansetron orally disintegrating Tablets are used for:
- preventing nausea and vomiting caused by chemotherapy or radiotherapy for cancer.
- preventing nausea and vomiting after surgery.
Ask your doctor, nurse, or pharmacist if you would like any further explanation about these uses.
2. What you need to know before you take Emigo®-MD Tablet
Do not take Emigo®-MD Tablet, if
- You are taking apomorphine (used to treat Parkinson's disease).
- You are allergic to ondansetron or any of the other ingredients of this medicine
If you are not sure, talk to your doctor, nurse, or pharmacist before taking Emigo®-MD Tablet.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before taking Emigo®-MD Tablets if:
- you are allergic to medicines similar to ondansetron known as 5HT3 antagonists such as granisetron or palonosetron.
- you suffer from severe constipation, or have you been told you have a blockage in your gut.
- you have ever had heart problems (e.g. congestive heart failure which causes shortness of breath and swollen ankles)
- you have an uneven heart beat (arrhythmias)
- you suffer from any liver problems.
- you have problems with the levels of salts in your blood, such as potassium, sodium and magnesium.
- if you have had an operation to remove both the adenoids and tonsils (adenotonsillar surgery).
If you are not sure if any of the above apply to you, talk to your doctor, nurse, or pharmacist before taking Emigo®-MD Tablets.
Other medicines and Emigo®-MD Tablets
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines that you buy without a prescription and herbal medicines. This is because ondansetron can affect the way some medicines work. Also some other medicines can affect the way ondansetron works. Emigo®-MD Tablets may have an effect on other drugs or other drugs may have an effect on Emigo®-MD Tablets.
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines.
- Anti-arrhythmic medicines such as amiodarone used to treat an uneven heartbeat.
- Phenytoin or carbamazepine, used to treat epilepsy.
- Rifampicin, an antibiotic used to treat infections.
- Antibiotics such as erythromycin or ketoconazole.
- Tramadol, a pain relieving medicine.
- Beta-blocker medicines used to treat certain heart or eye problems, anxiety or prevent migraines.
- Medicines that affect the heart (such as haloperidol or methadone).
- Cancer medicines (especially anthracyclines and trastuzumab).
- SSRIs (selective serotonin reuptake inhibitors) used to treat depression and/or anxiety including fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram, escitalopram.
- SNRIs (serotonin noradrenaline reuptake inhibitors) used to treat depression and/or anxiety including venlafaxine, duloxetine.
Contact your doctor. It may be necessary to adjust the dose.
Emigo®-MD Tablets with food and drink
You may take Ondansetron tablets with food and drinks. The tablets should be taken with a glass of water.
Children and adolescents
Children receiving Emigo®-MD Tablets with drugs that are harmful to liver should undergo regular check-up for liver problems.
Pregnancy and breast-feeding
If you are already pregnant or breast-feeding, think you might be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. If you are a woman of childbearing potential, you may be advised to use effective contraception.
Pregnancy
You should not use Emigo®-MD Tablets during the first trimester of pregnancy. This is because Emigo®-MD Tablets can slightly increase the risk of a baby being born with cleft lip and/or cleft palate (openings or splits in the upper lip and/or the roof of the mouth).
Breast-feeding
Do not take Emigo®-MD Tablets if you are breast-feeding, because it is excreted into the milk.
Driving and using machines
Ondansetron is unlikely to affect your ability to drive or operate machinery.
3. How to use Emigo®-MD Tablets
Always take Emigo®-MD Tablets exactly as your doctor has told you. Check with your doctor, nurse or pharmacist if you are not sure.
The dose you have been prescribed will depend on the treatment you are having.
To prevent nausea and vomiting from chemotherapy or radiotherapy
On the day of chemotherapy or radiotherapy
- The recommended adult dose is 8 mg taken one or two hours before treatment and another 8 mg twelve hours after.
On the following days
- The recommended adult dose is 8 mg twice a day
- This may be given for up to 5 days.
Children aged over 6 months and adolescents
The doctor will decide the dose depending on the child’s size (body surface area) or weight.
Look at the label for more information.
- The recommended dose for a child is up to 4 mg twice a day.
- This can be given for up to 5 days.
To prevent nausea and vomiting after an operation
The usual adult dose is 16 mg before your operation or
- 8 mg before the operation, then
- 8 mg after the operation, then
- 8 mg after a further eight hours.
Children aged over 1 month and adolescents.
It is recommended that ondansetron is given as an injection.
Patients with moderate or severe liver problems
The total daily dose should not be more than 8 mg.
Emigo®-MD Tablets should start to work within one or two hours of taking a dose.
If you are sick (vomit) within one hour of taking a dose
- Take the same dose again
- Otherwise, do not take more Emigo®-MD tablets than the label says.
If you continue to feel sick, tell your doctor or nurse.
If you take more Emigo®-MD Tablets than you should
If you or your child take more Emigo®-MD Tablets than you should, talk to a doctor or go to a hospital straight away. Take the medicine pack with you.
If you forget to take Ondansetron Tablets
If you miss a dose and feel sick or vomit:
- Take Emigo®-MD Tablets as soon as possible, then
- Take your next tablet at the usual time (as shown on the label)
- Do not take a double dose to make up for a forgotten dose.
If you miss a dose but do not feel sick
- Take the next dose as shown on the label
- Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Emigo®-MD can cause side effects, although not everybody gets them.
Allergic reactions
If you have an allergic reaction, stop taking it and see a doctor straight away. The signs may include:
- Sudden wheezing and chest pain or chest tightness
- Swelling of your eyelids, face, lips, mouth, or tongue
- Skin rash - red spots or lumps under your skin (hives) anywhere on your body
- Collapse
Other side effects include:
Very common (may affect more than 1 in 10 people)
- Headache
Common (may affect up to 1 in 10 people)
- A feeling of warmth or flushing
- Constipation
- Changes to liver function test results (if you take EMIGO MD® Tablets with a medicine called cisplatin, otherwise this side effect is uncommon)
Uncommon (may affect up to 1 in 100 people)
- Hiccups
- Low blood pressure, which can make you feel faint or dizzy
- Uneven heart beat
- Chest pain
- Fits
- Unusual body movements or shaking
- Slower heart rate
Rare (may affect up to 1 in 1,000 people)
- Feeling dizzy or light headed
- Blurred vision
- Disturbance in heart rhythm (sometimes causing a sudden loss of consciousness)
Very rare (may affect up to 1 in 10,000 people)
- Poor vision or temporary loss of eyesight, which usually comes back within 20 minutes
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Emigo®-MD tablets
- Keep this medicine out of the sight and reach of children.
- Do not use Emigo®-MD Tablets after the expiry date which is stated on the pack after ‘EXP’. The expiry date refers to the last day of that month.
- Store Emigo®-MD Tablets in the original blister to protect from light and moisture.
- Emigo®-MD Tablets should only be taken out of the blister immediately before taking them.
- If your doctor tells you to stop taking Emigo®-MD Tablets, it is important to return any which are left over to your pharmacist.
- Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
Emigo®-MD Tablets
Each uncoated mouth dissolving tablet contains:
Ondansetron IP 4 mg.
Excipients q.s.
Colour: Lake Erythrosine.
What Emigo®-4 looks like and contents of the pack Film-coated tablet.
Emigo®-4 contains 10 blister strips of 10 tablets each.