Enoxarin 40 & 60 Injection
Therapy Area
Cardiology
Composition
ENOXARIN 40
Each Pre-filled syringe contains :
Enoxaparin Sodium IP 40 mg
Water for Injections IP q.s. to 0.4 ml
ENOXARIN 60
Each Pre-filled syringe contains :
Enoxaparin Sodium IP 60 mg
Water for Injections IP q.s. to 0.6 ml
Description
ENOXARIN Injection is a sterile aqueous solutions containing Enoxaparin Sodium, a low molecular weight heparin. The average molecular weight is about 4,500 daltons.
Clinical Pharmacology
Pharmacodynamics
In humans, Enoxaparin given at a dose of 1.5 mg/kg subcutaneously (SC) is characterized by a higher ratio of anti-Factor Xa to anti-Factor IIa activity (mean±SD, 14.0±3.1) (based on areas under anti-Factor activity versus time curves) compared to the ratios observed for heparin (mean±SD, 1.22±0.13). Increases of up to 1.8 times the control values were seen in the thrombin time (TT) and the activated partial thromboplastin time (aPTT). Enoxaparin at a 1 mg/kg dose (100 mg/mL concentration), administered SC every 12 hours to patients in a large clinical trial resulted in aPTT values of 45 seconds or less in the majority of patients (n =1607). A 30 mg IV bolus immediately followed by a 1 mg/kg SC administration resulted in aPTT post-injection values of 50 seconds. The average aPTT prolongation value on Day 1 was about 16% higher than on Day 4.
Pharmacokinetics
Absorption : Pharmacokinetic trials were conducted using the 100 mg/mL formulation. Maximum anti Factor Xa and anti-thrombin (anti-Factor IIa) activities occur 3 to 5 hours after SC injection of Enoxaparin. Mean peak anti-Factor Xa activity was 0.16 IU/mL (1.58 mcg/mL) and 0.38 IU/mL (3.83 mcg/mL) after the 20 mg and the 40 mg clinically tested SC doses, respectively. Mean (n = 46) peak anti-Factor Xa activity was 1.1 IU/mL at steady state in patients with unstable angina receiving 1 mg/kg SC every 12 hours for 14 days. Mean absolute bioavailability of Enoxaparin, after 1.5 mg/kg given SC, based on anti-Factor Xa activity is approximately 100% in healthy subjects. A 30 mg IV bolus immediately followed by a 1 mg/kg SC every 12 hours provided initial peak anti-Factor Xa levels of 1.16 IU/mL (n=16) and average exposure corresponding to 84% of steady-state levels. Steady state is achieved on the second day of treatment. Enoxaparin pharmacokinetics appear to be linear over the recommended dosage ranges. After repeated subcutaneous administration of 40 mg once daily and 1.5 mg/kg once daily regimens in healthy volunteers, the steady state is reached on day 2 with an average exposure ratio about 15% higher than after a single dose. Steady-state Enoxaparin activity levels are well predicted by single-dose pharmacokinetics. After repeated subcutaneous administration of the 1 mg/kg twice daily regimen, the steady state is reached from day 4 with mean exposure about 65% higher than after a single dose and mean peak and trough levels of about 1.2 and 0.52 IU/mL, respectively. Based on Enoxaparin Sodium pharmacokinetics, this difference in steady state is expected and within the therapeutic range.
Distribution : The volume of distribution of anti-Factor Xa activity is about 4.3 L.
Elimination : Following intravenous (IV) dosing, the total body clearance of Enoxaparin is 26 mL/min. After IV dosing of Enoxaparin labeled with the gamma-emitter, 99m Tc, 40% of radioactivity and 8 to 20% of anti-Factor Xa activity were recovered in urine in 24 hours. Elimination half-life based on antiFactor Xa activity was 4.5 hours after a single SC dose to about 7 hours after repeated dosing. Significant anti-Factor Xa activity persists in plasma for about 12 hours following a 40 mg SC once a day dose. Following SC dosing, the apparent clearance (CL/F) of Enoxaparin is approximately 15 mL/min.
Metabolism : Enoxaparin Sodium is primarily metabolized in the liver by desulfation and/or depolymerization to lower molecular weight species with much reduced biological potency. Renal clearance of active fragments represents about 10% of the administered dose and total renal excretion of active and non-active fragments 40% of the dose.
Indications :
Prophylaxis of Deep Vein Thrombosis
Enoxaparin Sodium Injection is indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE)
- In patients undergoing abdominal surgery who are at risk for thromboembolic complications.
- In patients undergoing hip replacement surgery, during and following hospitalization.
- In patients undergoing knee replacement surgery.
- In medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness.
Treatment of Acute Deep Vein Thrombosis
Enoxaparin Sodium Injection is indicated for
- The inpatient treatment of acute deep vein thrombosis with or without pulmonary embolism, when administered in conjunction with warfarin Sodium.
- The outpatient treatment of acute deep vein thrombosis without pulmonary embolism when administered in conjunction with warfarin Sodium.
Prophylaxis of Ischemic Complications of Unstable Angina and Non-Q-Wave Myocardial Infarction
Treatment of Acute ST-Segment Elevation Myocardial Infarction
Contraindications:
- Active major bleeding
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of Enoxaparin Sodium
- Known hypersensitivity to Enoxaparin Sodium (e.g., pruritus, urticaria, anaphylactic/ anaphylactoid reactions)
- Known hypersensitivity to heparin or pork products
Dosage & Administration :
Abdominal Surgery : In patients undergoing abdominal surgery who are at risk for thromboembolic complications, the recommended dose of Enoxaparin Sodium Injection is 40 mg once a day administered by SC injection with the initial dose given 2 hours prior to surgery. The usual duration of administration is 7 to 10 days; up to 12 days administration has been administered in clinical trials.
Hip or Knee Replacement Surgery : In patients undergoing hip or knee replacement surgery, the recommended dose of Enoxaparin Sodium Injection is 30 mg every 12 hours administered by SC injection. Provided that hemostasis has been established, the initial dose should be given 12 to 24 hours after surgery. For hip replacement surgery, a dose of 40 mg once a day SC, given initially 12 (±3) hours prior to surgery, may be considered. Following the initial phase of thromboprophylaxis in hip replacement surgery patients, it is recommended that continued prophylaxis with Enoxaparin Sodium Injection 40 mg once a day be administered by SC injection for 3 weeks. The usual duration of administration is 7 to 10 days; up to 14 days administration has been administered in clinical trials.
Medical Patients During Acute Illness : In medical patients at risk for thromboembolic complications due to severely restricted mobility during acute illness, the recommended dose of Enoxaparin Sodium Injection is 40 mg once a day administered by SC injection. The usual duration of administration is 6 to 11 days; up to 14 days of Enoxaparin Sodium Injection has been administered in the controlled clinical trial.
Treatment of Deep Vein Thrombosis with or without Pulmonary Embolism : In outpatient treatment, patients with acute deep vein thrombosis without pulmonary embolism who can be treated at home, the recommended dose of Enoxaparin Sodium Injection is 1 mg/kg every 12 hours administered SC. In inpatient (hospital) treatment, patients with acute deep vein thrombosis with pulmonary embolism or patients with acute deep vein thrombosis without pulmonary embolism (who are not candidates for outpatient treatment), the recommended dose of Enoxaparin Sodium Injection is 1 mg/kg every 12 hours administered SC or 1.5 mg/kg once a day administered SC at the same time every day. In both outpatient and inpatient (hospital) treatments, warfarin Sodium therapy should be initiated when appropriate (usually within 72 hours of Enoxaparin Sodium Injection). Enoxaparin Sodium Injection should be continued for a minimum of 5 days and until a therapeutic oral anticoagulant effect has been achieved (International Normalization Ratio 2.0 to 3.0). The average duration of administration is 7 days; up to 17 days of Enoxaparin Sodium Injection administration has been administered in controlled clinical trials.
Unstable Angina and Non-Q-Wave Myocardial Infarction : In patients with unstable angina or non-Q-wave myocardial infarction, the recommended dose of Enoxaparin Sodium Injection is 1 mg/kg administered SC every 12 hours in conjunction with oral aspirin therapy (100 to 325 mg once daily). Treatment with Enoxaparin Sodium Injection should be prescribed for a minimum of 2 days and continued until clinical stabilization. The usual duration of treatment is 2 to 8 days; up to 12.5 days of Enoxaparin Sodium Injection has been administered in clinical trials.
Treatment of Acute ST-Segment Elevation Myocardial Infarction : In patients with acute ST-segment elevation myocardial infarction, the recommended dose of Enoxaparin Sodium Injection is a single IV bolus of 30 mg plus a 1 mg/kg SC dose followed by 1 mg/kg administered SC every 12 hours (maximum 100 mg for the first two doses only, followed by 1 mg/kg dosing for the remaining doses). Dosage adjustments are recommended in patients ≥75 years of age. All patients should receive aspirin as soon as they are identified as having STEMI and maintained with 75 to 325 mg once daily unless contraindicated. When administered in conjunction with a thrombolytic (fibrin-specific or non-fibrin specific), Enoxaparin Sodium Injection should be given between 15 minutes before and 30 minutes after the start of fibrinolytic therapy. In the pivotal clinical study, the Enoxaparin Sodium Injection treatment duration was 8 days or until hospital discharge, whichever came first. An optimal duration of treatment is not known, but it is likely to be longer than 8 days. For patients managed with percutaneous coronary intervention (PCI) : If the last Enoxaparin Sodium Injection SC administration was given less than 8 hours before balloon inflation, no additional dosing is needed. If the last Enoxaparin Sodium Injection SC administration was given more than 8 hours before balloon inflation, an IV bolus of 0.3 mg/kg of Enoxaparin Sodium Injection should be administered.
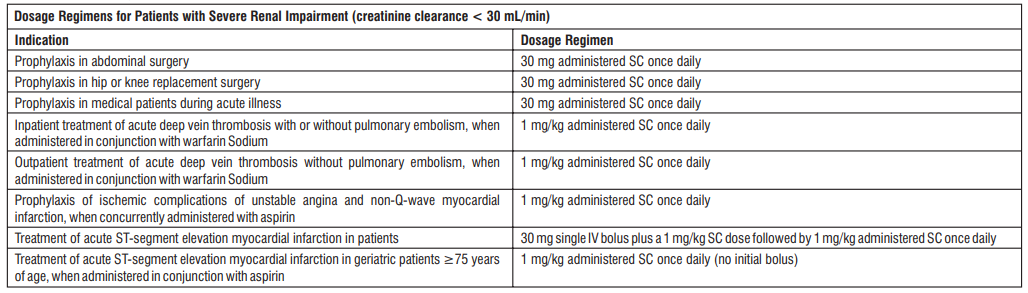
Renal impairment : Although no dose adjustment is recommended in patients with moderate (creatinine clearance 30–50 mL/min) and mild (creatinine clearance 50–80 mL/min) renal impairment, all such patients should be observed carefully for signs and symptoms of bleeding. The recommended prophylaxis and treatment dosage regimens for patients with severe renal impairment (creatinine clearance < 30 mL/min) are described in the table below
Recommended Dosage for Geriatric Patients with Acute ST-Segment Elevation Myocardial Infarction
For treatment of acute ST-segment elevation myocardial infarction in geriatric patients ≥75 years of age, do not use an initial intravenous bolus. Initiate dosing with 0.75 mg/kg subcutaneously every 12 hours (maximum 75 mg for the first two doses only, followed by 0.75 mg/kg dosing for the remaining doses). No dose adjustment is necessary for other indications in geriatric patients unless kidney function is impaired.
Adverse Effects
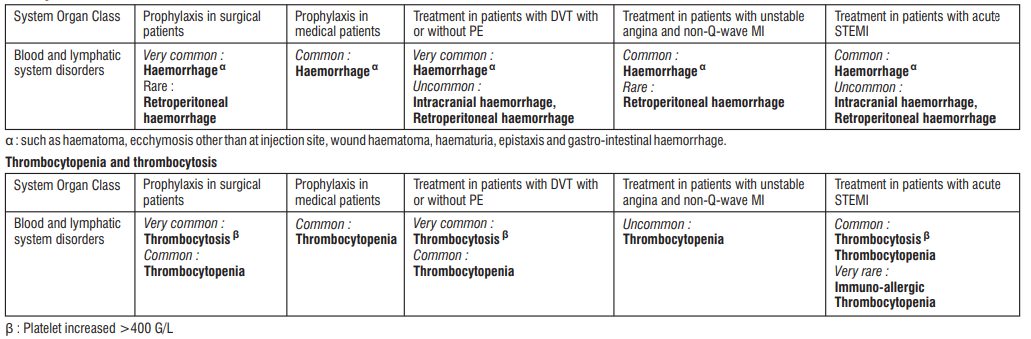
In clinical studies, haemorrhages, thrombocytopenia and thrombocytosis were the most commonly reported reactions Other adverse reactions observed in clinical studies and reported in post-marketing experience (* indicates reactions from post-marketing experience) are detailed below. Frequencies are defined as follows : very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1000 to < 1/100); rare (≥ 1/10,000 to <1/1,000); and very rare (< 1/10,000) or not known (cannot be estimated from available data). Within each system organ class, adverse reactions are presented in order of decreasing seriousness.
Blood and the lymphatic system disorders
- Common : Haemorrhage, haemorrhagic anaemia*, thrombocytopenia, thrombocytosis
- Rare : Eosinophilia*, cases of immuno-allergic thrombocytopenia with thrombosis; in some of them thrombosis was complicated by organ infarction or limb ischaemia (see section 4.4).
Immune system disorders
- Common : Allergic reaction
- Rare : Anaphylactic/Anaphylactoid reactions including shock*
Nervous system disorders
- Common : Headache*
Vascular disorders
- Rare : Spinal haematoma* (or neuraxial haematoma). These reactions have resulted in varying degrees of neurologic injuries including long-term or permanent paralysis (see section 4.4).
Hepato-biliary disorders
- Very common : Hepatic enzyme increases (mainly transaminases > 3 times the upper limit of normality)
- Uncommon : Hepatocellular liver injury *
- Rare : Cholestatic liver injury*
Skin and subcutaneous tissue disorders
- Common : Urticaria, pruritus, erythema
- Uncommon : Bullous dermatitis
- Rare : Alopecia, cutaneous vasculitis*, skin necrosis* usually occurring at the injection site (these phenomena have been usually preceded by purpura or erythematous plaques, infiltrated and painful).
- Injection site nodules* (inflammatory nodules, which were not cystic enclosure of Enoxaparin). They resolve after a few days and should not cause treatment discontinuation.
Musculoskeletal, connective tissue and bone disorders
- Rare : Osteoporosis* following long term therapy (greater than 3 months)
General disorders and administration site conditions
- Common : Injection site haematoma, injection site pain, other injection site reaction (such as oedema, haemorrhage, hypersensitivity, inflammation, mass, pain, or reaction)
- Uncommon : Local irritation, skin necrosis at injection site
Investigations
- Rare : Hyperkalaemia
Description of selected adverse reactions
Haemorrhages
These included major haemorrhages, reported at most in 4.2% of the patients (surgical patients). Some of these cases have been fatal. In surgical patients, haemorrhage complications were considered major : (1) if the haemorrhage caused a significant clinical event, or (2) if accompanied by haemoglobin decrease ≥ 2 g/dL or transfusion of 2 or more units of blood products. Retroperitoneal and intracranial haemorrhages were always considered major. As with other anticoagulants, haemorrhage may occur in the presence of associated risk factors such as : organic lesions liable to bleed, invasive procedures or the concomitant use of medications affecting haemostasis.
Warnings and Precautions
Increased Risk of Hemorrhage
- Cases of epidural or spinal hemorrhage and subsequent hematomas have been reported with the use of Enoxaparin Sodium Injection and epidural or spinal anesthesia/analgesia or spinal puncture procedures, resulting in long-term or permanent paralysis. The risk of these events is higher with the use of postoperative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity.
- Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of Enoxaparin is low. Placement or removal of a catheter should be delayed for at least 12 hours after administration of lower doses (30 mg once or twice daily or 40 mg once daily) of Enoxaparin Sodium Injection, and at least 24 hours after the administration of higher doses (0.75 mg/kg twice daily, 1 mg/kg twice daily, or 1.5 mg/kg once daily) of Enoxaparin Sodium Injection.
- Patients receiving the 0.75 mg/kg twice daily dose or the 1 mg/kg twice daily dose should not receive the second Enoxaparin dose in the twice daily regimen to allow a longer delay before catheter placement or removal. Likewise, although a specific recommendation for timing of a subsequent Enoxaparin Sodium Injection dose after catheter removal cannot be made, consider delaying this next dose for at least four hours, based on a benefit-risk assessment considering both the risk for thrombosis and the risk for bleeding in the context of the procedure and patient risk factors.
- For patients with creatinine clearance <30mL/minute, additional considerations are necessary because elimination of Enoxaparin is more prolonged; consider doubling the timing of removal of a catheter, at least 24 hours for the lower prescribed dose of Enoxaparin Sodium Injection (30 mg once daily) and at least 48 hours for the higher dose (1 mg/kg/day).
- Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction
- Instruct patients to report immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
- Enoxaparin Sodium injection should be used with extreme caution in conditions with increased risk of hemorrhage, such as bacterial endocarditis, congenital or acquired bleeding disorders, active ulcerative and angiodysplastic gastrointestinal disease, hemorrhagic stroke, or shortly after brain, spinal, or ophthalmological surgery, or in patients treated concomitantly with platelet inhibitors. Major hemorrhages including retroperitoneal and intracranial bleeding have been repor ted. Some of these cases have been fatal. Bleeding can occur at any site during therapy with Enoxaparin Sodium injection. An unexplained fall in hematocrit or blood pressure should lead to a search for a bleeding site.
Percutaneous Coronary Revascularization Procedures
To minimize the risk of bleeding following the vascular instrumentation during the treatment of unstable angina, non-Q-wave myocardial infarction, and acute ST-segment elevation myocardial infarction, adhere precisely to the intervals recommended between Enoxaparin Sodium injection doses. It is important to achieve hemostasis at the puncture site after PCI. In case a closure device is used, the sheath can be removed immediately. If a manual compression method is used, sheath should be removed 6 hours after the last IV/SC Enoxaparin Sodium injection. If the treatment with Enoxaparin Sodium is to be continued, the next scheduled dose should be given no sooner than 6 to 8 hours after sheath removal. The site of the procedure should be observed for signs of bleeding or hematoma formation.
Use of Enoxaparin Sodium Injection with Concomitant Medical Conditions
Enoxaparin Sodium injection should be used with care in patients with a bleeding diathesis, uncontrolled arterial hypertension or a history of recent gastrointestinal ulceration, diabetic retinopathy, renal dysfunction and hemorrhage
History of Heparin-Induced Thrombocytopenia
Enoxaparin Sodium injection should be used with extreme caution in patients with a history of heparin induced thrombocytopenia.
Thrombocytopenia
Thrombocytopenia can occur with the administration of Enoxaparin Sodium injection. Thrombocytopenia of any degree should be monitored closely. If the platelet count falls below 100,000/mm3, Enoxaparin Sodium injection should be discontinued. Cases of heparin-induced thrombocytopenia with thrombosis have also been observed in clinical practice. Some of these cases were complicated by organ infarction, limb ischemia, or death
Interchangeability with Other Heparins
Enoxaparin Sodium injection cannot be used interchangeably (unit for unit) with heparin or other low molecular weight heparins as they differ in manufacturing process, molecular weight distribution, anti- Xa and anti-IIa activities, units, and dosage. Each of these medicines has its own instructions for use.
Pregnant Women with Mechanical Prosthetic Heart Valves
The use of Enoxaparin Sodium injection for thromboprophylaxis in pregnant women with mechanical prosthetic heart valves has not been adequately studied. There also have been isolated postmarketing reports of valve thrombosis in pregnant women with mechanical prosthetic heart valves while receiving Enoxaparin for thromboprophylaxis. Women with mechanical prosthetic heart valves may be at higher risk for thromboembolism during pregnancy, and, when pregnant, have a higher rate of fetal loss from stillbirth, spontaneous abortion and premature delivery. Therefore, frequent monitoring of peak and trough anti- Factor Xa levels, and adjusting of dosage may be needed.
Laboratory Tests
Periodic complete blood counts, including platelet count, and stool occult blood tests are recommended during the course of treatment with Enoxaparin Sodium injection. When administered at recommended prophylaxis doses, routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT) are relatively insensitive measures of Enoxaparin Sodium injection activity and, therefore, unsuitable for monitoring. Anti-Factor Xa may be used to monitor the anticoagulant effect of Enoxaparin Sodium injection in patients with significant renal impairment. If during Enoxaparin Sodium injection therapy abnormal coagulation parameters or bleeding should occur, anti-Factor Xa levels may be used to monitor the anticoagulant effects of Enoxaparin Sodium injection.
Use in Special Population
Pregnancy
Pregnancy Category B
All pregnancies have a background risk of birth defect, loss, or other adverse outcome regardless of drug exposure. The fetal risk summary below describes the potential of Enoxaparin Sodium injection to increase the risk of developmental abnormalities above the background risk.
Fetal Risk Summary
Enoxaparin Sodium Injection does not cross the placenta, and is not expected to result in fetal exposure to the drug. Human data suggest that Enoxaparin Sodium Injection does not increase the risk of major developmental abnormalities. Based on animal data, Enoxaparin is not predicted to increase the risk of major developmental abnormalities.
Nursing Mothers
It is not known whether Enoxaparin Sodium injection is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Enoxaparin Sodium injection, a decision should be made whether to discontinue nursing or discontinue Enoxaparin Sodium injection, taking into account the importance of Enoxaparin Sodium injection to the mother and the known benefits of nursing.
Pediatric Use
Safety and effectiveness of Enoxaparin Sodium injection in pediatric patients have not been established.
Geriatric Use
Prevention of Deep Vein Thrombosis in Hip, Knee and Abdominal Surgery; Treatment of Deep Vein Thrombosis, Prevention of Ischemic Complications of Unstable Angina and Non-Q-wave
Myocardial Infarction
The incidence of bleeding complications was higher in geriatric patients as compared to younger patients when Enoxaparin Sodium injection was administered at doses of 1.5 mg/kg once a day or 1 mg/kg every 12 hours. The risk of Enoxaparin Sodium injection-associated bleeding increased with age. Serious adverse events increased with age for patients receiving Enoxaparin Sodium injection. Careful attention to dosing intervals and concomitant medications (especially antiplatelet medications) is advised. Enoxaparin Sodium injection should be used with care in geriatric patients who may show delayed elimination of Enoxaparin. Monitoring of geriatric patients with low body weight (<45 kg) and those predisposed to decreased renal function should be considered
Treatment of Acute ST-Segment Elevation Myocardial Infarction
In the clinical study for treatment of acute ST-segment elevation myocardial infarction, there was no evidence of difference in efficacy between patients ≥75 years of age (n = 1241) and patients less than 75 years of age (n = 9015). Patients ≥75 years of age did not receive a 30 mg IV bolus prior to the normal dosage regimen and had their SC dose adjusted to 0.75 mg/kg every 12 hours. The incidence of bleeding complications was higher in patients ≥65 years of age as compared to younger patients (<65 years).
Renal Impairment
In patients with renal impairment, there is an increase in exposure of Enoxaparin Sodium. All such patients should be observed carefully for signs and symptoms of bleeding. Because exposure of Enoxaparin Sodium is significantly increased in patients with severe renal impairment (creatinine clearance <30 mL/min), a dosage adjustment is recommended for therapeutic and prophylactic dosage ranges. No dosage adjustment is recommended in patients with moderate (creatinine clearance 30–50 mL/min) and mild (creatinine clearance 50–80 mL/min) renal impairment. In patients with renal failure, treatment with Enoxaparin has been associated with the development of hyperkalemia.
Hepatic Impairment
The impact of hepatic impairment on Enoxaparin's exposure and antithrombotic effect has not been investigated. Caution should be exercised when administering Enoxaparin to patients with hepatic impairment.
Low-Weight Patients
An increase in exposure of Enoxaparin Sodium with prophylactic dosages (non-weight adjusted) has been observed in low-weight women (<45 kg) and low-weight men (<57 kg). All such patients should be observed carefully for signs and symptoms of bleeding.
Obese Patients
Obese patients are at higher risk for thromboembolism. The safety and efficacy of prophylactic doses of Enoxaparin Sodium Injection in obese patients (BMI >30 kg/m ) has not been fully determined and there is no consensus for dose adjustment. These patients should be observed carefully for signs and symptoms of thromboembolism.
Drug Interactions
Whenever possible, agents which may enhance the risk of hemorrhage should be discontinued prior to initiation of Enoxaparin Sodium injection therapy. These agents include medications such as : anticoagulants, platelet inhibitors including acetylsalicylic acid, salicylates, NSAIDs (including ketorolac tromethamine), dipyridamole, or sulfinpyrazone. If co-administration is essential, conduct close clinical and laboratory monitoring
Overdosage
Accidental overdosage following administration of Enoxaparin Sodium injection may lead to hemorrhagic complications. Injected Enoxaparin Sodium injection may be largely neutralized by the slow IV injection of protamine sulfate (1% solution). The dose of protamine sulfate should be equal to the dose of Enoxaparin Sodium injection injected : 1 mg protamine sulfate should be administered to neutralize 1 mg Enoxaparin Sodium injection, if Enoxaparin Sodium was administered in the previous 8 hours. An infusion of 0.5 mg protamine per 1 mg of Enoxaparin Sodium may be administered if Enoxaparin Sodium was administered greater than 8 hours previous to the protamine administration, or if it has been determined that a second dose of protamine is required. The second infusion of 0.5 mg protamine sulfate per 1 mg of Enoxaparin Sodium injection may be administered if the aPTT measured 2 to 4 hours after the first infusion remains prolonged. If at least 12 hours have elapsed since the last Enoxaparin Sodium injection, protamine administration may not be required; however, even with higher doses of protamine, the aPTT may remain more prolonged than following administration of heparin. In all cases, the anti-Factor Xa activity is never completely neutralized (maximum about 60%). Particular care should be taken to avoid overdosage with protamine sulfate. Administration of protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal reactions, often resembling anaphylaxis, have been reported with protamine sulfate, it should be given only when resuscitation techniques and treatment of anaphylactic shock are readily available.
Storage
Store below 25°C. Do not freeze
Keep out of reach of children
Shelf-life
Refer on the pack
Presentation :
ENOXARIN 40 : A pre-filled syringe of 40 mg / 0.4 ml.
ENOXARIN 60 : A pre-filled syringe of 60 mg / 0.6 ml.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What ENOXARIN® is and what it is used for
2. What you need to know before you take ENOXARIN®
3. How to take ENOXARIN®
4. Possible side effects
5. How to store ENOXARIN®
6. Contents of the pack and other information
1. What ENOXARIN® is and what it is used for
ENOXARIN® contains the active substance called enoxaparin sodium that is a low molecular weight heparin (LMWH).
ENOXARIN® works in two ways:
- Stopping existing blood clots from getting any bigger. This helps your body to break them down and stops them from causing you harm
- Stopping blood clots from forming in your blood.
ENOXARIN® can be used to:
- Treat blood clots that are in your blood.
- Stop blood clots from forming in your blood in the following situations:
- Before and after an operation
- When you have an acute illness and face period of limited mobility
- After a heart attack
- Stop blood clots forming in the tubes of your dialysis machine (used for people with severe kidney problems).
2. What you need to know before you take ENOXARIN®
Do not take ENOXARIN®,
- If you are allergic to enoxaparin sodium or any of the other ingredients of this medicine. Signs of an allergic reaction include: rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue.
- If you are allergic to heparin or other low molecular weight heparins such as nadroparin, tinzaparin or dalteparin.
- If you have had a reaction to heparin that caused a severe drop in the number of your clotting cells (platelets) - this reaction is called heparin-induced thrombocytopenia - within the last 100 days or if you have antibodies against enoxaparin in your blood.
- If you are bleeding heavily or have a condition with a high risk of bleeding (such as stomach ulcer, recent surgery of the brain or eyes), including recent bleeding stroke.
- If you are using ENOXARIN® to treat blood clots in your body and going to receive spinal or epidural anaesthesia or lumbar puncture within 24 hours.
Tell your doctor if any of the above applies to you before this medicine is used.
Warnings and precautions
ENOXARIN® should not be used interchangeably with other medicines belonging to the group of low molecular weight heparins. This is because they are not exactly the same and do not have the same activity and instructions for use.
Talk to your doctor or pharmacist before using ENOXARIN® if:
- you have ever had a reaction to heparin that caused a severe drop in the number of your platelets
- you are going to receive spinal or epidural anaesthesia or lumbar puncture: a delay should be respected between ENOXARIN® use and this procedure
- you have had a heart valve fitted
- you have endocarditis (an infection of the inner lining of the heart)
- you have history of gastric ulcer
- you have had a recent stroke
- you have high blood pressure
- you have diabetes or problems with blood vessels in the eye caused by diabetes (called diabetic retinopathy)
- you have had an operation recently on your eyes or brain
- you are elderly (over 65 years old) and especially if you are over 75 years old
- you have kidney problems
- you have liver problems
- you are underweight or overweight
- you have high level of potassium in your blood (this may be checked with a blood test)
- you are currently using medicines which affect bleeding
You may have a blood test before you start using this medicine and at intervals while you are using it; this is to check the level of the clotting cells (platelets) and potassium in your blood.
Other medicines and ENOXARIN®
Tell your doctor or pharmacist if you are taking or might take/use any other medicines.
Warfarin – used for thinning the blood
Aspirin (also known as acetylsalicylic acid or ASA), clopidogrel or other medicines used to stop blood clots from forming
Dextran injection – used as a blood replacer
Ibuprofen, diclofenac, ketorolac or other medicines known as non-steroidal anti-inflammatory agents which are used to treat pain and swelling in arthritis and other conditions
Prednisolone, dexamethasone or other medicines used to treat asthma, rheumatoid arthritis and other conditions
Medicines which increase potassium level in your blood such as potassium salts, water pills, some medicines for heart problems.
Operations and Anaesthetics
If you are going to have a spinal puncture or an operation where an epidural or spinal anaesthetic is used, tell you doctor that you are using ENOXARIN®. Also, tell your doctor if you have any problem with your spine or if you ever had spinal surgery
Pregnancy and breast-feeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. If you are pregnant and have a mechanical heart valve, you may be at an increased risk of developing blood clots. Your doctor should discuss this with you. If you are breast-feeding or plan to breast-feed, you should ask your doctor for advice before taking this medicine.
Driving and using machines
ENOXARIN® does not affect the ability to drive and operate machinery.
3. How to use ENOXARIN®
- Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
- Having this medicine
- Your doctor or nurse will normally give you ENOXARIN®. This is because it needs to be given as an injection.
- When you go home, you may need to continue to use ENOXARIN® and give it yourself.
- ENOXARIN® is usually given by injection underneath the skin (subcutaneous).
- ENOXARIN® can be given by injection into your vein (intravenous) after certain types of heart attack or operation.
- ENOXARIN® can be added to the tube leaving the body (arterial line) at the start of the dialysis session.
Do not inject ENOXARIN® into a muscle.
How much will be given to you
Your doctor will decide how much ENOXARIN® to give you. The amount will depend on the reason it is being used.
If you have problems with your kidneys you may be given a smaller amount of ENOXARIN®.
1. Treating blood clots that are in your blood
- The usual dose is 150 IU (1.5 mg) for every kilogram of your weight each day or 100 IU/mg (1 mg) for every kilogram of your weight twice a day.
Your doctor will decide how long you should receive ENOXARIN®.
2. Stopping blood clots forming in your blood in the following situations:
Operation or periods of limited mobility due to an illness
- The dose will depend on how likely you are to develop a clot. You will be given 2,000 IU (20 mg) or 4,000 IU (40 mg) of ENOXARIN® each day.
- If you are going to have an operation your first injection will be usually given 2 hours or 12 hours before your operation.
- If you have restricted mobility due to illness, you will normally be given 4,000 IU (40 mg) of ENOXARIN® each day.
- Your doctor will decide how long you should receive ENOXARIN®.
After you have had a heart attack
ENOXARIN® can be used for heart attack called as STEMI (ST segment elevation myocardial infarction). The amount of ENOXARIN® given to you will depend on your age and the kind of heart attack you have had.
STEMI type of heart attack if you are under 75 years old:
An initial dose of 3,000 IU (30 mg) of ENOXARIN® will be given as injection into your vein.
At the same time you will also be given ENOXARIN® as an injection underneath your skin (subcutaneous injection). The usual dose is 100 IU (1 mg) for every kilogram of your weight, every 12 hours.
Your doctor will normally ask you to take aspirin (acetylsalicylic acid) as well.
Your doctor will decide how long you should receive ENOXARIN®. STEMI type of heart attack if you are 75 years old or older:
The usual dose is 75 IU (0.75 mg) for every kilogram of your weight, every 12 hours.
The maximum amount of ENOXARIN® given for the first two injections is 7,500 IU (75 mg).
Your doctor will decide how long you should receive ENOXARIN®. For patients that have an operation called percutaneous coronary intervention
(PCI):
Depending on when you were last given ENOXARIN®, your doctor may decide to give an additional dose of ENOXARIN® before a PCI operation. This is by injection into your vein.
3. Stopping blood clots from forming in the tubes of your dialysis machine
The usual dose is 100 IU (1 mg) for every kilogram of your weight.
ENOXARIN® is added to the tube leaving the body (arterial line) at the start of the dialysis session. This amount is usually enough for a 4-hour session. However, your doctor may give you a further dose of 50 IU to 100 IU (0.5 to 1 mg) for every kilogram of your weight, if necessary.
Instructions for use of the syringe
How to give yourself an injection of ENOXARIN®
If you are able to give ENOXARIN® to yourself, your doctor or nurse will show you how to do this. Do not try to inject yourself if you have not been trained how to do so. If you are not sure what to do, talk to your doctor or nurse immediately. Performing the injection properly
under the skin (called “subcutaneous injection”) will help reduce pain and bruising at the injection site.
Before injecting yourself with ENOXARIN®
- Collect together the items that you need: syringe, alcohol swab or soap and water, and sharps container.
- Check the expiry date on the medicine. Do not use if the date has passed.
- Check the syringe is not damaged and the medicine in it is a clear solution. If not, use another syringe.
- Make sure you know how much you are going to inject.
- Check your abdomen to see if the last injection caused any redness, change in skin colour, swelling, oozing or is still painful. If so talk to your doctor or nurse.
Changing of anticoagulant treatment
Changing from ENOXARIN® to blood thinners called vitamin-K antagonists (e.g. warfarin) Your doctor will request you perform blood tests called INR and tell you when to stop ENOXARIN® accordingly.
Changing from blood thinners called vitamin-K antagonists (e.g. warfarin) to ENOXARIN®
Stop taking the vitamin-K antagonist. Your doctor will request you perform blood tests called INR and tell you when to start ENOXARIN® accordingly.
Changing from ENOXARIN® to treatment with direct oral anticoagulant Stop taking ENOXARIN®. Start taking the direct oral anticoagulant 0-2 hours before the time you would have had the next injection, then continue as normal.
Changing from treatment with direct oral anticoagulant to ENOXARIN® Stop taking direct oral anticoagulant. Do not start treatment with ENOXARIN® until 12 hours after the final dose of direct oral anticoagulant.
Use in children and adolescents
The safety and efficacy of ENOXARIN® has not been evaluated in children or adolescents.
If you use more ENOXARIN® than you should
If you think that you have used too much or too little ENOXARIN®, tell your doctor, nurse or pharmacist immediately, even if you have no signs of a problem. If a child accidentally injects or swallows ENOXARIN®, take them to a hospital casualty department straight away.
If you forget to use ENOXARIN®
If you forget to give yourself a dose, have it as soon as you remember. Do not give yourself a double dose on the same day to make up for a forgotten dose. Keeping a diary will help to make sure you do not miss a dose.
If you stop using ENOXARIN®
If you have any further questions on the use of this medicine, ask your doctor or pharmacist or nurse.
It is important for you to keep having ENOXARIN® injections until your doctor decides to stop them. If you stop, you could get a blood clot which can be very dangerous.
4. Possible side effects
Like other similar medicines (medicines to reduce blood clotting), ENOXARIN® may cause bleeding which may potentially be life-threatening. In some cases, the bleeding may not be obvious.
If you experience any bleeding event that does not stop by itself or if you experience signs of excessive bleeding (exceptional weakness, tiredness, paleness, dizziness, headache or unexplained swelling), consult your doctor immediately.
Your doctor may decide to keep you under closer observation or change your medicine. Stop using ENOXARIN® and talk to a doctor or nurse at once if you get any signs of a severe allergic reaction (such as difficulty breathing, swelling of the lips, mouth, throat or eyes).
You should tell your doctor straight away
- If you have any sign of blockage of a blood vessel by a blood clot such as:
- cramping pain, redness, warmth, or swelling in one of your legs – these are symptoms of deep vein thrombosis
- breathlessness, chest pain, fainting or coughing up blood – these are symptoms of a pulmonary embolism
- If you have a painful rash of dark red spots under the skin which do not go away when you put pressure on them.
Your doctor may request you perform a blood test to check your platelet count.
Overall list of possible side effects:
Very common (may affect more than 1 in 10 people)
- Bleeding
- Increases in liver enzymes.
Common (may affect up to 1 in 10 people)
- You bruise more easily than usual. This could be because of a blood problem with low platelet counts
- Pink patches on your skin. These are more likely to appear in the area you have been injected with ENOXARIN®.
- Skin rash (hives, urticaria).
- Itchy red skin.
- Bruising or pain at the injection site.
- Decreased red blood cell count.
- High platelet counts in the blood.
- Headache
Uncommon (may affect up to 1 in 100 people)
- Sudden severe headache. This could be a sign of bleeding in the brain.
- A feeling of tenderness and swelling in your stomach. You may have bleeding in your stomach.
- Large red irregularly shaped skin lesions with or without blisters.
- Skin irritation (local irritation).
- You notice yellowing of your skin or eyes and your urine becomes darker in colour. This could be a liver problem.
Rare (may affect up to 1 in 1,000 people)
- Severe allergic reaction. The signs may include: a rash, swallowing or breathing problems, swelling of your lips, face, throat or tongue.
- Increased potassium in your blood. This is more likely to happen in people with kidney problems or diabetes. Your doctor will be able to check this by carrying out a blood test.
- Hair loss.
- Osteoporosis (a condition where your bones are more likely to break) after long term use.
- Tingling, numbness and muscular weakness (particularly in the lower part of your body) when you have had a spinal puncture or a spinal anaesthetic.
- Lost of control over your bladder or bowel (so you cannot control when you go to the toilet).
- Hard mass or lump at the injection site.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store ENOXARIN®
Store below 25°C. Do not freeze.
ENOXARIN® prefilled syringes are single dose containers – discard any unused product. Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the label. The expiry date refers to the last day of that month. Do not use this medicine if you notice the syringe is damaged or the product is not clear. Medicines should not be disposed via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
ENOXARIN® 40 mg Injection
Each pre-filled syringe contains:
Enoxaparin Sodium IP 40 mg
Water for Injection IP q.s to 0.4 ml.
ENOXARIN® 60 mg Injection
Each pre-filled syringe contains:
Enoxaparin Sodium IP 60 mg
Water for Injection IP q.s to 0.6 ml.