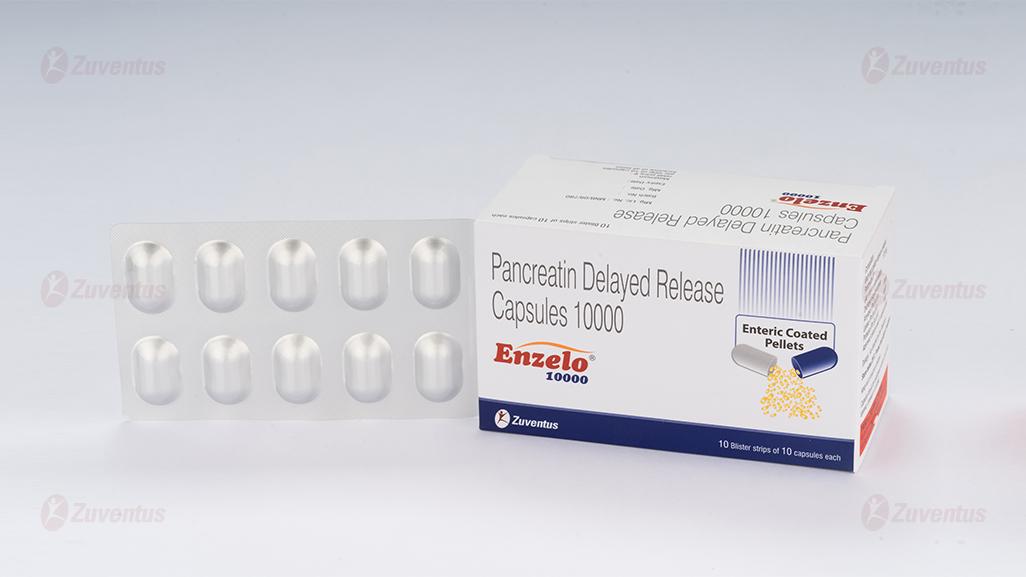
Enzelo 10000 Capsules
Therapy Area
Gastrointestinal
1.0 Generic name
Pancreatin
2.0 Qualitative and quantitative composition
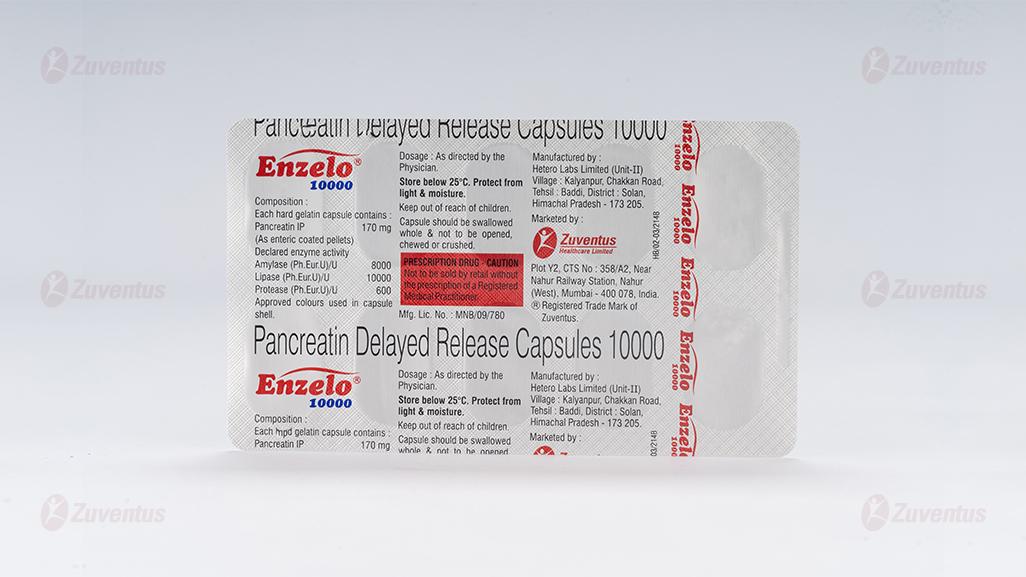
ENZELO 10000
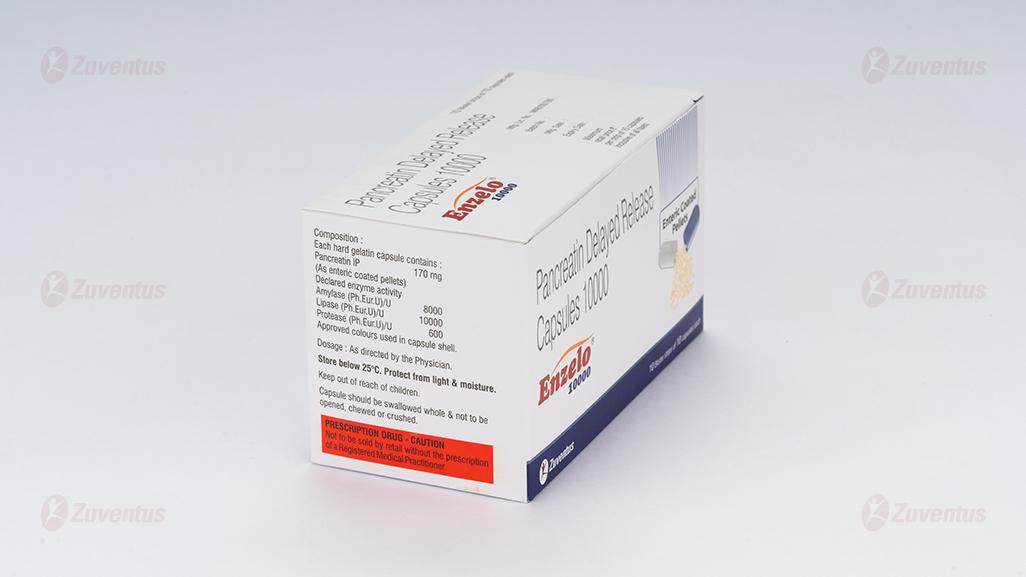
Each hard gelatin capsule contains:
Pancreatin IP 170 mg
(As enteric coated pellets)
Declared enzyme activity
Amylase (Ph.Eur.U)/U 8000
Lipase (Ph.Eur.U)/U 10000
Protease (Ph.Eur.U)/U 600
Approved Colours used in capsule shell
ENZELO 25000
Each hard gelatin capsule contains: Pancreatin IP 350 mg
(As enteric coated pellets)
Declared enzyme activity
Amylase (Ph.Eur.U)/U 18000
Lipase (Ph.Eur.U)/U 25000
Protease (Ph.Eur.U)/U 1000
Approved Colours used in capsule shell
3.0 Dosage form and strength
Capsule
Pancreatin 170 mg / 350 mg
4.0 Clinical particulars
4.1 Therapeutic Indication
For the treatment of pancreatic exocrine insufficiency
4.2 Posology and method of administration
Adults (including the elderly) and children:
Initially one or two capsules during each meal. Dose increases, if required, should be added slowly, with careful monitoring of response and symptomatology.
It is important to ensure adequate hydration of patients at all times whilst dosing Enzelo 25000.
Fibrosing colonopathy has been reported in patients with cystic fibrosis taking in excess of 10,000 units of lipase/kg/day
Method of administration
Oral use.
Capsule should be swallowed whole & not to be opened, chewed or crushed.
4.3 Contraindications
Hypersensitivity to Pancreatin of porcine origin or to any of the excipients
4.4 Special warnings and precautions for use
Strictures of the ileo-caecum and large bowel (fibrosing colonopathy) have been reported in patients with cystic fibrosis taking high doses of pancreatin preparations. As a precaution, unusual abdominal symptoms or changes in abdominal symptoms should be medically assessed to exclude the possibility of fibrosing colonopathy, especially if the patient is taking in excess of 10,000 units of lipase/kg/day.
4.5 Drugs interactions
No interaction studies have been performed.
4.6 Use in special populations
Pregnancy
For pancreatic enzymes no clinical data on exposed pregnancies are available.
Animal studies show no evidence for any absorption of porcine pancreatic enzymes. Therefore, no reproductive or developmental toxicity is to be expected.
Caution should be exercised when prescribing to pregnant women.
Nursing Mothers
No effects on the suckling child are anticipated since animal studies suggest no systemic exposure of the breast-feeding woman to pancreatic enzymes. Pancreatic enzymes can be used during breast-feeding.
If required during pregnancy or lactation Enzelo should be used in doses sufficient to provide adequate nutritional status.
4.7 Effects on ability to drive and use machines
Enzelo has no or negligible influence on the ability to drive or use machines.
4.8 Undesirable effects
| Organ system | Very common ≥ 1/10 | Common ≥ 1/100 to < 1/10 | Uncommon ≥ 1/1000 to < 1/100 | Frequency not known |
|---|---|---|---|---|
| Gastrointestinal disorders | abdominal pain* | nausea, vomiting, constipation, abdominal distention, diarrhoea* | strictures of the ileo-caecum and large bowel (fibrosing colonopathy) | |
| Skin and subcutaneous tissue disorders | rash | pruritus, urticaria | ||
| Immune system disorders | hypersensitivity (anaphylactic reactions). |
*Gastrointestinal disorders are mainly associated with the underlying disease. Similar or lower incidences compared to placebo were reported for abdominal pain and diarrhoea.
Strictures of the ileo-caecum and large bowel (fibrosing colonopathy) have been reported in patients with cystic fibrosis taking high doses of pancreatin preparations, see section 4.4 Special warnings and precautions for use.
Allergic reactions mainly but not exclusively limited to the skin have been observed and identified as adverse reactions during post-approval use. Because these reactions were reported spontaneously from a population of uncertain size, it is not possible to reliably estimate their frequency.
Paediatric population
No specific adverse reactions were identified in the paediatric population. Frequency, type and severity of adverse reactions were similar in children with cystic fibrosis as compared to adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
4.9 Overdose
Extremely high doses of pancreatin have been reported to be associated with hyperuricosuria and hyperuricaemia.
Supportive measures including stopping enzyme therapy and ensuring adequate rehydration are recommended.
5.0 Pharmacological properties
5.1 Mechanism of Action
The capsules dissolve rapidly in the stomach releasing plenty of minimicrospheres, a multidose principle which is designed to achieve good mixing with the chyme, emptying from the stomach together with the chyme and after release, good distribution of enzymes within the chyme.
When the minimicrospheres reach the small intestine the coating rapidly disintegrates (at pH > 5.5) to release enzymes with lipolytic, amylolytic and proteolytic activity to ensure the digestion of fats, starches and proteins. The products of pancreatic digestion are then either absorbed directly, or following further hydrolysis by intestinal enzymes.
5.2 Pharmacodynamic properties
Treatment with enzelo markedly improves the symptoms of pancreatic exocrine insufficiency including stool consistency, abdominal pain, flatulence and stool frequency, independent of the underlying disease.
5.3 Pharmacokinetic properties
Pharmacokinetic data are not available as the enzymes act locally in the gastro-intestinal tract. After exerting their action, the enzymes are digested themselves in the intestine.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Not applicable.
7.0 Description
Pancreatin is a pancreatic enzyme supplement, containing Lipase, Amylase, Protease
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on Pack
8.3 Packaging information
10 Blister strips of 10 capsules each
8.4 Storage and handing instructions
Store below 25°C.
Protect from light & moisture.
Keep out of reach of children.
9.0 Patient counselling information
- Capsule should be swallowed whole & not to be opened, chewed or crushed.
- Capsule should be taken with the first bite of a meal
- Should be taken with sufficient fluid, adequate hydration should be ensured
- Dosage should not be changed or increased without the physician direction
- Sporadic doses (not taken with food) should be avoided
- Do not take this medicine, if you are allergic to porcine pancreatin.
- Signs of an allergic reaction include a rash, itching or shortness of breath.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
- If you have any further questions, ask your doctor or pharmacist.
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only.
- Do not pass it on to others.
- It may harm them, even if their signs of illness are the same as yours.
- ENZELO is a pancreatic enzyme supplement for people whose bodies do not make enough enzymes to digest their food.
- Take the number of capsules as prescribed by your doctor or dietician.
- ENZELO Capsule should be taken with the first bite of a meal or a snack and drink plenty of water.
- Do not take ENZELO if you are allergic to pork or any pig product.
- If you experience severe abdominal pain while taking ENZELO, contact your doctor immediately.
- Most people do not have problems taking ENZELO but side effects can occur.
- If you get any side effects, talk to your doctor, pharmacist or nurse.
- This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What ENZELO is and what it is used for
- What you need to know before you take ENZELO
- How to take ENZELO
- Possible side effects
- How to store ENZELO
- Contents of the pack and other information
1. What Enzelo is and What It is Used for
What is ENZELO
- ENZELO is a high strength pancreatic enzyme supplement.
- Pancreatic enzyme supplements are used by people whose bodies do not make enough of their own enzymes to digest their food.
- ENZELO granules contain a mixture of the natural enzymes which are used to digest food.
- The enzymes are taken from pig pancreas glands.
How ENZELO works
The enzymes in ENZELO work by digesting food as it passes through the gut. So you must take ENZELO at the same time as eating a meal or a snack. This will allow the enzymes to mix thoroughly with the food.
2. What You Need to Know Before You Take Enzelo
Do not use ENZELO
you are allergic to pork, any pig product, or to any of the other ingredients If the above applies to you do not take ENZELO. Talk to your doctor or dietician again.
Warnings and precautions
A rare bowel condition called “fibrosing colonopathy”, where gut is narrowed, has been reported in patients with cystic fibrosis taking high dose pancreatin products.
If you have cystic fibrosis and take in excess of 10,000 lipase units per kilogram per day and have unusual abdominal symptoms or changes in abdominal syptoms tell you doctor.
Talk to your doctor if:
- You are pregnant or trying to get pregnant (ENZELO can be used while breast feeding)
- Please tell your doctor dietician or pharmacist if you think that you should not take ENZELO for any other reason.
If you drive or use machines
It is unlikely that ENZELO will affect your ability to drive or operate tools or machines.
3. How to Use Enzelo
How much ENZELO to take
- Always follow your doctor or dietician’s advice on how many capsules to take.
- Initially one or two capsules during each meal.
- It is important to ensure adequate hydration of patients at all times whilst dosing Enzelo 25000.
- If your doctor advises you to increase the number of capsules you take, you should do so slowly with careful monitoring of response and symptomatology. If you still have fatty stools or abdominal pain, talk to your doctor or dietician.
When to take ENZELO
- ENZELO Capsule should be taken with the first bite of a meal or a snack and drink plenty of water
How to take ENZELO
- Swallow the capsules whole
- Drink plenty of liquid every day.
- Mixing with non-acidic food or liquid, crushing or chewing of the pellets may cause irritation in your mouth or change the way ENZELO works in your body.
- Do not hold ENZELO capsules or its content in your mouth.
How long to take ENZELO for?
You should take your medicine until your doctor tells you to stop. Many patients will need to take pancreatic enzymes supplements for the rest of their lives.
If you take too much ENZELO
If you take too much ENZELO you should drink plenty of water and see your doctor immediately.
If you forget a dose
If you forget to take your medicine, wait until your next meal and take your usual number of capsules.
Do not try to make up for the number of capsules that you have missed. Just take your next dose at the usual time.
4. Possible Side Effects
Like all medicines, ENZELO can cause side effects (unwanted effects or reactions), but not everyone gets them.
If you have severe or long-lasting abdominal pain, contact your doctor immediately.
If you notice any unusual abdominal symptoms while taking ENZELO– contact your doctor.
Very common side effects (affect more than 1 in 10 patients):
- stomach pains
Common side effects (affect 1-10 patients of 100):
- diarrhoea
- constipation
- feeling or being sick
- bloating
Uncommon side effects (affect 1-10 patients of 1000):
- Skin reaction, such as a rash.
During use, some patients have also experienced the following, the frequency of which is unknown:
- itching with or without a rash
- allergic reactions (which may be severe)
- severe or long-lasting abdominal pain (Fibrosing colonopathy).
At extremely high doses, some patients have had high levels of uric acid in their blood and urine.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to Store Enzelo
Store below 25°C.
Protect from light & moisture.
Keep out of reach of children.
6. Contents of the Pack and Other Information
What ENZELO contains
ENZELO 10000
Each hard gelatin capsule contains:
Pancreatin IP 170 mg
(As enteric coated pellets)
Declared enzyme activity
Amylase (Ph.Eur.U)/U 8000
Lipase (Ph.Eur.U)/U 10000
Protease (Ph.Eur.U)/U 600
Approved Colours used in capsule shell
ENZELO 25000
Each hard gelatin capsule contains: Pancreatin IP 350 mg
(As enteric coated pellets)
Declared enzyme activity Amylase (Ph.Eur.U)/U 18000
Lipase (Ph.Eur.U)/U 25000
Protease (Ph.Eur.U)/U 1000
Approved Colours used in capsule shell
Packaging:
10 Blister strips of 10 capsules each