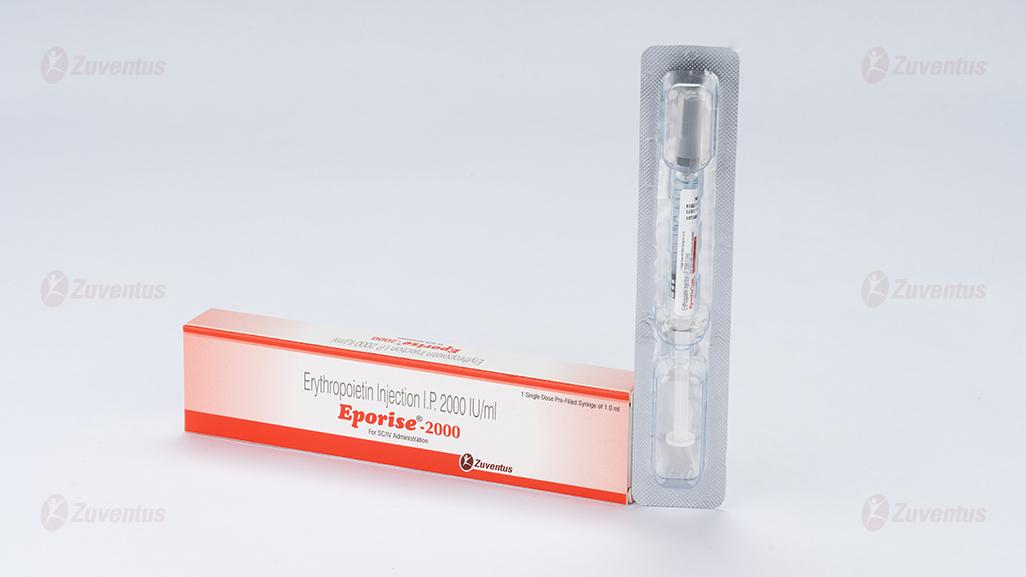
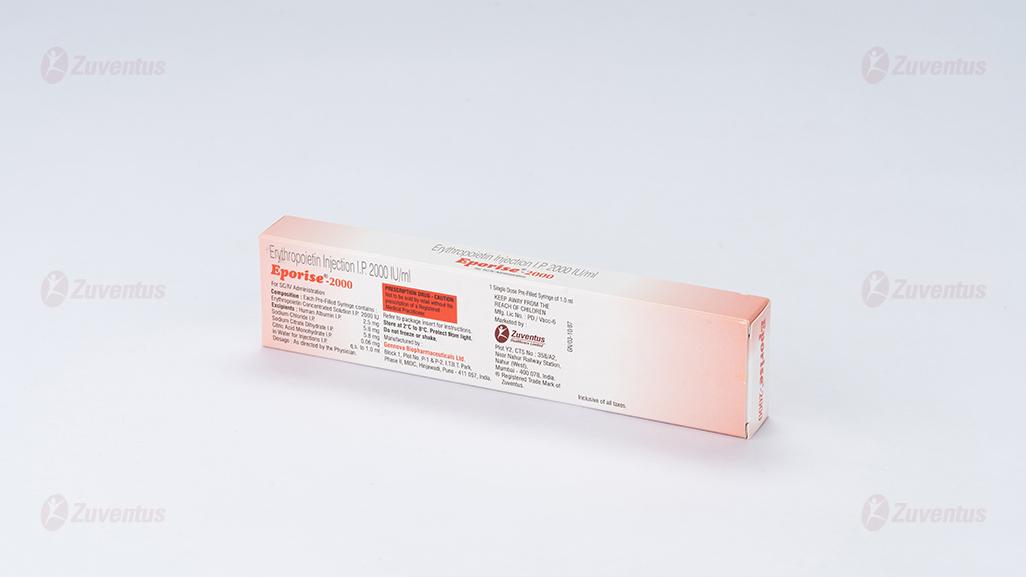
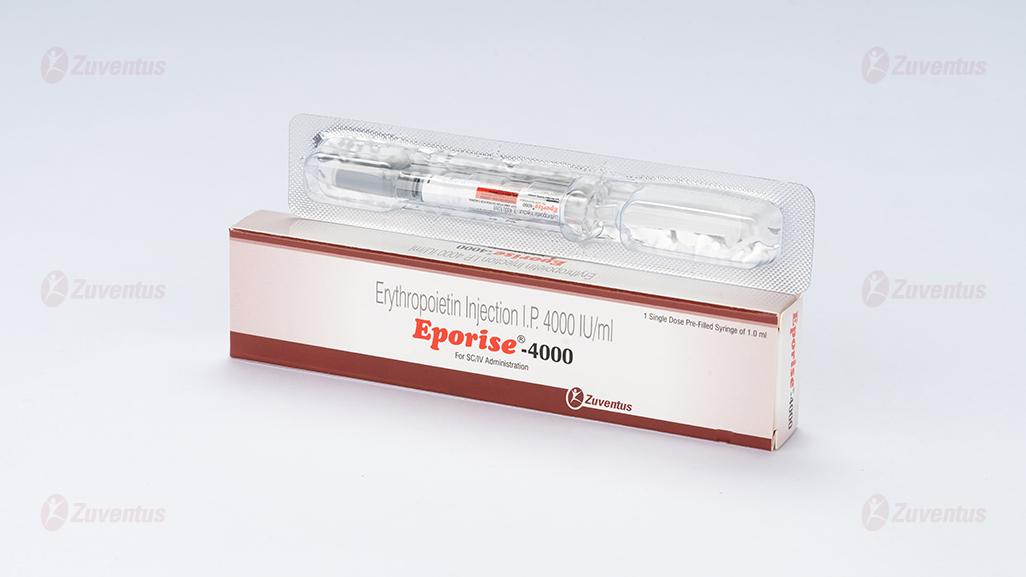
Eporise Injection
Therapy Area
Blood Related
For SC/IV Administration

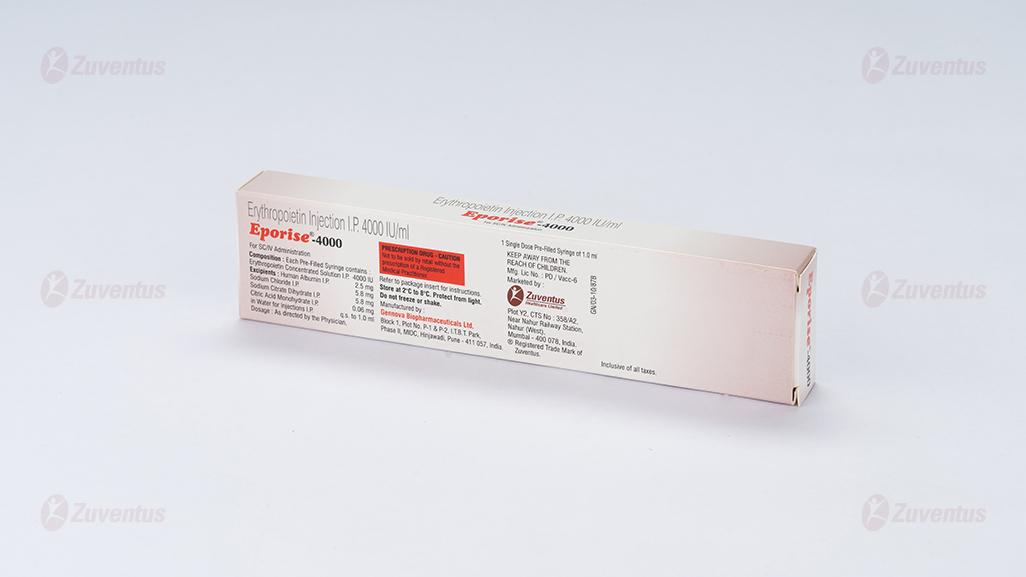
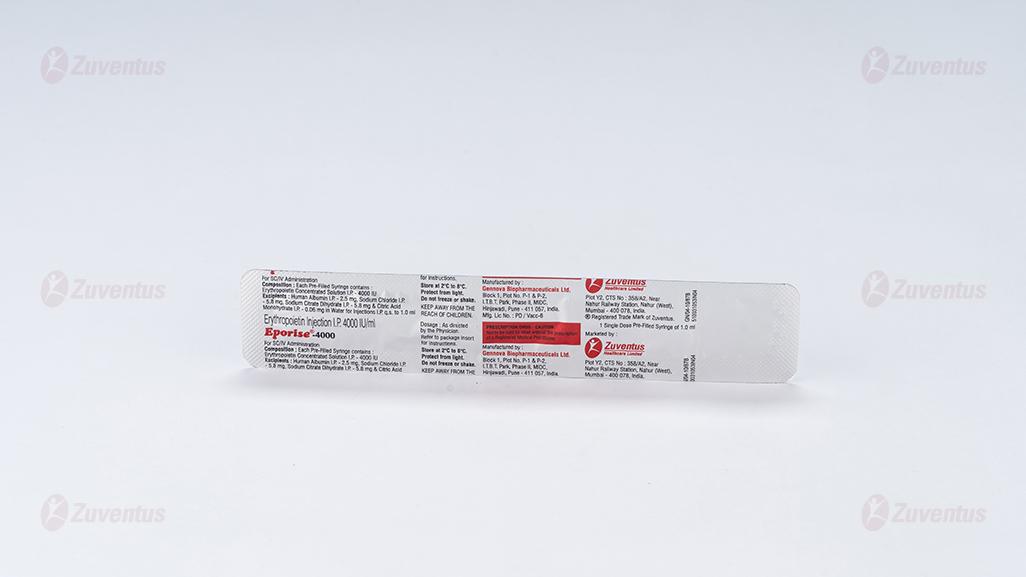
Composition

Pharmacodynamics

Zidovudine-treated HIV-infected patients
Dose Increase


Use In Special Population


Read all of this leaflet carefully before you start taking this medicine because it contains important information for you:
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours
- . If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Eporise® is and what it is used for
2. What you need to know before you take Eporise®
3. How to take Eporise®
4. Possible side effects
5. How to store Eporise®
6. Contents of the pack and other information
1. What Eporise® is and what it is used for
Eporise® contains the active substance Erythropoietin - a protein that stimulates the bone marrow to produce more red blood cells which carry haemoglobin (a substance that transports oxygen).
Eporise® is used to treat symptomatic anaemia caused by kidney disease
- in children on haemodialysis
- in adults on haemodialysis or peritoneal dialysis
- in severely anaemic adults not yet undergoing dialysis.
If you have kidney disease, you may be short of red blood cells if your kidney does not produce enough erythropoietin (necessary for red cell production). Eporise® is prescribed to stimulate your bone marrow to produce more red blood cells.
- Eporise® is used to treat anaemia in adults receiving chemotherapy for solid tumours, malignant lymphoma or multiple myeloma (bone marrow cancer) who may have a need for a blood transfusion. Eporise® can reduce the need for a blood transfusion in these patients.
- Eporise® is used in moderately anaemic adults who donate some of their blood before surgery, so that it can be given back to them during or after the operation. Because Eporise® stimulates the production of red blood cells, doctors can take more blood from these people.
- Eporise® is used in treating anaemia in HIV-infected person treated with Zidovudine. In these patients, Eporise® maintain the Red blood cell level and can reduce the need for a blood transfusion in these patients
- Eporise® is used to treat anaemia in premature infants (a baby which is born too early). Eporise® is prescribed to stimulate the production of red blood cells.
2. What you need to know before you take Eporise®
Do not use Eporise®
- If you are allergic to erythropoietin or any of the other ingredients of this medicine (listed in section 6).
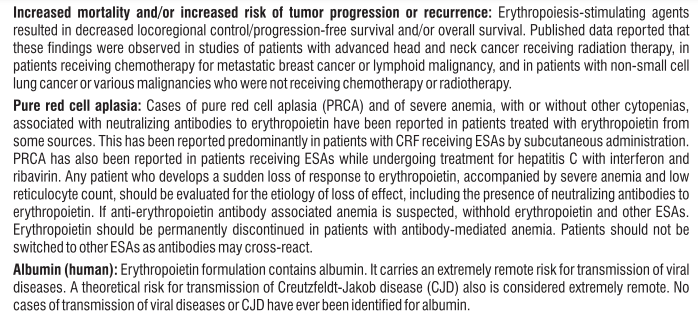
- If you have been diagnosed with Pure Red Cell Aplasia (the bone marrow cannot produce enough red blood cells) after previous treatment with any product that stimulates red blood cell production (including Eporise®). See section 4, Possible side effects.
- If you have high blood pressure not properly controlled with medicines.
- To stimulate the production of your red blood cells (so that doctors can take more blood from you) if you cannot have transfusions with your own blood during or after surgery.
- If you are due to have major elective orthopaedic surgery (such as hip or knee surgery), and you:
- have severe heart disease
- have severe disorders of the veins and arteries
- have recently had a heart attack or stroke
- can’t take medicines to thin the blood
Eporise® may not be suitable for you. Please discuss with your doctor. While on Eporise®, some people need medicines to reduce the risk of blood clots. If you can’t take medicines that prevent blood clotting, you must not have Eporise®
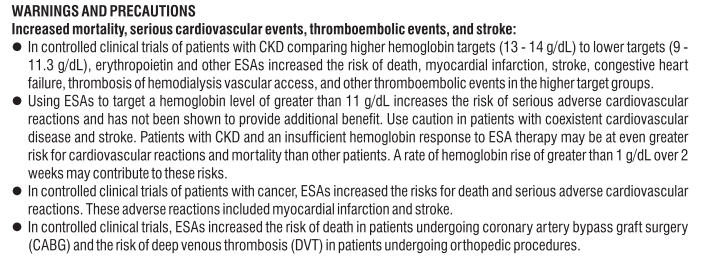
Warnings and precautions
Take special care with Eporise®
Eporise® and other products that stimulate red cell production may increase the risk of developing blood clots in all patients. This risk may be higher if you have other risk factors for developing blood clots (for example, if you have had a blood clot in the past or are overweight, have diabetes, have heart disease or you are off your feet for a long time because of surgery or illness). Please tell your doctor about any of these things. Your doctor will help you to decide if Eporise® is suitable for you.
It is important to tell your doctor if any of the following apply to you. You may still be able to use Eporise®, but discuss it with your doctor first.
If you know you suffer, or have suffered, from:
- high blood pressure;
- epileptic seizures or fits
- liver disease
- anaemia from other causes
- porphyria (a rare blood disorder)
- an allergy to latex. The needle cover of this medicinal product contains latex rubber which may cause severe allergic reactions in people who are sensitive to latex. See section 4 for the signs of an allergic reaction.
If you are a patient with chronic renal failure, and particularly if you do not respond properly to Eporise®, your doctor will check your dose of Eporise® because repeatedly increasing your dose of Eporise® if you are not responding to treatment may increase the risk of having a problem of the heart or the blood vessels and could increase risk of myocardial infarction, stroke and death.
If you are a cancer patient be aware that products that stimulate red blood cell production (like Eporise®) may act as a growth factor and therefore in theory may affect the progression of your cancer. Depending on your individual situation a blood transfusion may be preferable. Please discuss this with your doctor.
If you are a cancer patient, be aware that use of Eporise® may be associated with shorter survival and a higher death rate in head and neck, and metastatic breast cancer patients who are receiving chemotherapy.
Serious skin reactions including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported in association with epoetin treatment. SJS/TEN can appear initially as reddish target-like spots or circular patches often with central blisters on the trunk. Also, ulcers of mouth, throat, nose, genitals and eyes (red and swollen eyes) can occur. These serious skin rashes are often preceded by fever and/or flu-like symptoms. The rashes may progress to widespread peeling of the skin and life-threatening complications.
If you develop a serious rash or another of these skin symptoms, stop taking Eporise® and contact your doctor or seek medical attention immediately.
Take special care with other products that stimulate red blood cell production:
EPORISE® is one of a group of products that stimulate the production of red blood cells like the human protein erythropoietin does. Your healthcare professional will always record the exact product you are using.
If you are given a product in this group other than Eporise® during your treatment, speak to your doctor or pharmacist before using it.
Other medicines and Eporise®
Tell your doctor if you are taking, have recently taken or might take any other medicines.
If you are taking a drug called cyclosporin (used e.g. after kidney transplants), your doctor may order blood tests to check the level of cyclosporin while you are taking Eporise®.
Iron supplements and other blood stimulants may increase the effectiveness of Eporise®. Your doctor will decide if it is right for you to take them.
If you visit a hospital, clinic or family doctor, tell them you are having Eporise® treatment. It may affect other treatments or test results.
Pregnancy and breast-feeding
It is important to tell your doctor if any of the following apply to you. You may still be able to use Eporise®, but discuss it with your doctor first.
• If you are pregnant, or think you may be pregnant.
• If you are breastfeeding.
3. How to use Eporise®
Always use this medicine exactly as your doctor has told you. Check with your doctor if you are not sure.
Your doctor has carried out blood tests and decided you need Eporise®.
Eporise® may be given by injection:
• Either into a vein or a tube that goes into a vein (intravenously)
• Or under the skin (subcutaneously).
Your doctor will decide how Eporise® will be injected. The injections will be given to you by a doctor, nurse or other health care professional.
Eporise® should not be used:
• after the expiry date on the label and outer carton
• if you know, or think that it may have been accidentally frozen, or
• if there has been a refrigerator failure.
The dose of Eporise® you receive is based on your body weight in kilograms. The cause of your anaemia is also a factor in your doctor deciding the correct dose.
Your doctor will monitor your blood pressure regularly while you are using Eporise®.
People with kidney disease
• Your doctor will maintain your haemoglobin level between 10 and 12 g/dL as a high haemoglobin level may increase the risk of blood clots and death. In children the haemoglobin level should be maintained between 9.5 and 11 g/dL.
• The usual starting dose of Eporise® for adults and children is 50 to 100 International Units (IU) per kilogram (/kg) of bodyweight given three times a week.
• For adults and children Eporise® is given as an injection either into a vein or a tube that goes into a vein. When this access (via a vein or tube) is not readily available, your doctor may decide that Eporise® should be injected under the skin (subcutaneously). This includes patients on dialysis and patients not yet on dialysis.
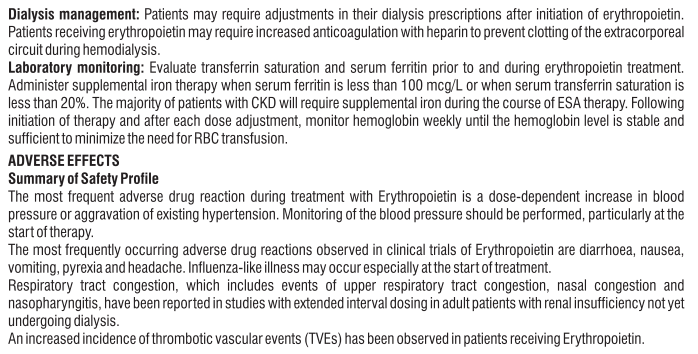
• Your doctor will order regular blood tests to see how your anaemia is responding and may adjust the dose, usually no more frequently than every four weeks. A rise in haemoglobin of greater than 2 g/dL over a four-week period should be avoided.
• Once your anaemia has been corrected, your doctor will continue to check your blood regularly. Your Eporise® dose and frequency of administration may be further adjusted to maintain your response to treatment. Your doctor will use the lowest effective dose to control the symptoms of your anemia.
• If you do not respond adequately to Eporise®, your doctor will check your dose and will Inform you if you need to change doses of Eporise®.
• If you are on a more extended dosing interval (greater than once weekly) of Eporise®, you May not maintain adequate haemoglobin levels and you may require an increase in Eporise®, dose or frequency of administration. • You may be given iron supplements before and during Eporise®, treatment to make it more effective.
• If you are having dialysis treatment when you begin treatment with Eporise®, your dialysis regime may need to be adjusted. Your doctor will decide this. Adults on chemotherapy
• Your doctor may initiate treatment with Eporise® if your haemoglobin is 10 g/dL or less.
• Your doctor will maintain your haemoglobin level between 10 and 12 g/dL as a high haemoglobin level may increase the risk of blood clots and death.
• The starting dose is either 150 IU per kilogram bodyweight three times a week or 40000 IU once a week.
• Eporise® is given by injection under the skin.
• Your doctor will order blood tests, and may adjust the dose, depending on how your anaemia responds to Eporise® treatment.
• You may be given iron supplements before and during Eporise® treatment to make it more effective.
• You will usually continue Eporise® treatment for one month after the end of chemotherapy
Adults donating their own blood
• The usual dose is 300 IU per kilogram bodyweight per day.
• Eporise® is given by injection into a vein immediately after you have donated blood for 3 weeks before your surgery.
• You may be given iron supplements before and during Eporise® treatment to make it more effective.
Adults scheduled for major orthopaedic surgery
• The recommended dose is 600 IU per kilogram bodyweight once a week.
• Eporise® is given by injection under the skin each week for three weeks before surgery and on the day of surgery.
• If there is a medical need to reduce the time before your operation, you will be given a daily dose of 300 IU/kg for up to ten days before surgery, on the day of surgery and for four days immediately afterwards.
• If blood tests show your haemoglobin is too high before the operation, the treatment will be stopped.
• You may be given iron supplements before and during Eporise® treatment to make it more effective.
Zidovudine-treated HIV-infected patients:
Starting dose: 100 IU/kg as an IV or SC injection three times a week for 8 weeks. Maintenance dose:
- If hemoglobin does not increase after 8 weeks of therapy, increase erythropoietin dose by approximately 50 to 100 IU/kg at 4- to 8-week intervals until haemoglobin reaches a level needed to avoid RBC transfusions or 300 IU/kg dose is reached.
- If the hemoglobin exceeds 12 g/dL, the dose should be discontinued.
- Resume therapy at a dose 25% below the previous dose when hemoglobin declines to less than 11 g/dL.
- Discontinue erythropoietin if an increase in hemoglobin is not achieved at a dose of 300 IU/kg for 8 weeks.
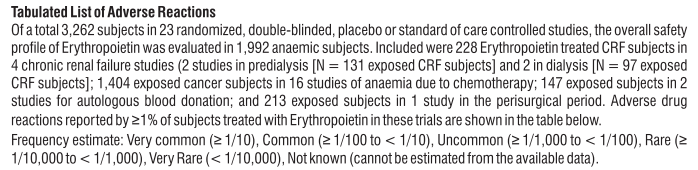
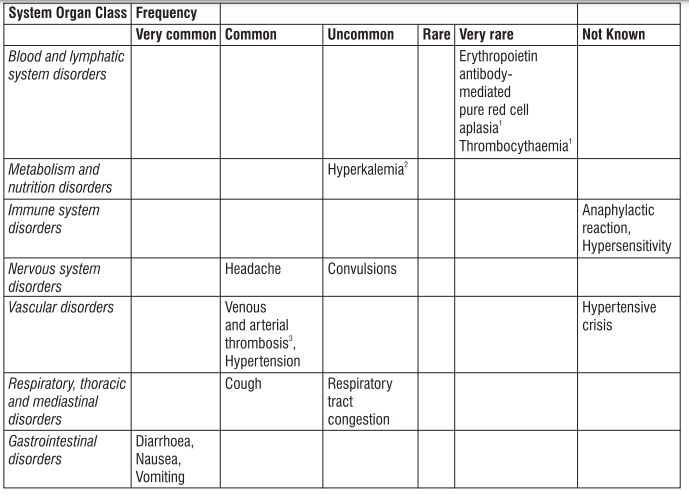
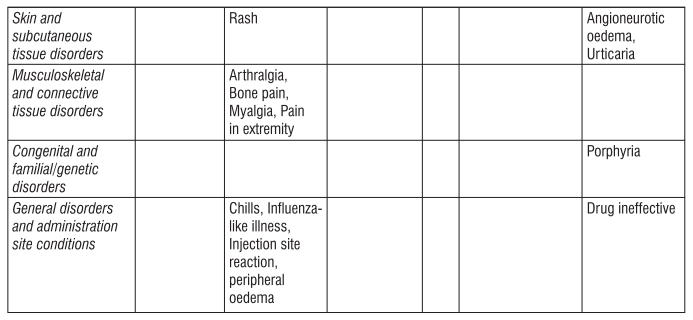
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or nurse immediately if you notice any of the effects in this list.
Serious skin rashes including Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported in association with epoetin treatment. These can appear as reddish target-like macules or circular patches often with central blisters on the trunk, skin peeling, ulcers of mouth, throat, nose, genitals and eyes and can be preceded by fever and flu-like symptoms. Stop using Eporise® if you develop these symptoms and contact your doctor or seek medical attention immediately. See also section 2.
Very common side effects
These may affect more than 1 in 10 people
• Diarrhoea
• Feeling sick in your stomach
• Vomiting
• Fever
• Respiratory tract congestion, such as stuffy nose and sore throat, has been reported in patients with kidney disease not yet on dialysis.
Common side effects
These may affect up to 1 in 10 people
• Increased blood pressure. Headaches, particularly sudden, stabbing migraine-like headaches, feeling confused or having fits may be signs of a sudden increase in blood pressure. This requires urgent treatment. Raised blood pressure may require treatment with drugs (or adjustment to any drugs you already take for high blood pressure).
• Blood clots (including deep vein thrombosis and embolism) that may require urgent treatment. You may have chest pain, breathlessness, and painful swelling and redness, usually in the leg as symptoms.
• Cough
• Skin rashes, which may result from an allergic reaction.
• Bone or muscle pain
• Flu-like symptoms, such as headache, aches and pains in the joints, feeling of weakness, chills, tiredness and dizziness. These may be more common at the start of treatment. If you have these symptoms during injection into the vein, a slower delivery of the injection may help to avoid them in the future.
• Redness, burning and pain at the site of injection
• Swelling of the ankles, feet or fingers
• Arm or leg pain
Uncommon side effects
These may affect up to 1 in 100 people
• High levels of blood potassium which can cause abnormal heart rhythm (this is a very common side effect in patients on dialysis).
• Fits
• Nose or airway congestion
• Allergic reaction
• Hives Rare side effects These may affect up to 1 in 1,000 people
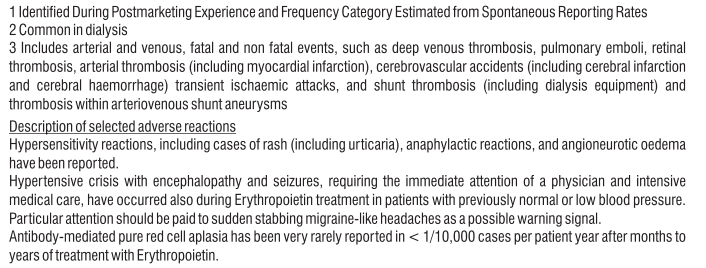
• Symptoms of pure red cell aplasia (PRCA) PRCA means the bone marrow does not make enough red blood cells. PRCA causes sudden and severe anaemia. The symptoms are:
• unusual tiredness,
• feeling dizzy,
• breathlessness.
PRCA has been very rarely reported mostly in patients with kidney disease after months to years of treatment with Eporise® and other products that stimulate red blood cell production
An increase in levels of small blood cells (called platelets), which are normally involved in the formation of a blood clot may occur, particularly when starting treatment. Your doctor will check on this.
• Severe allergic reaction that may include:
- a swollen face, lips, mouth, tongue or throat
- difficulty swallowing or breathing
- itchy rash (hives)
• Problem with the blood that may cause pain, dark-coloured urine or increased sensitivity of the skin to sunlight (porphyria)
If you are receiving haemodialysis:
• Blood clots (thrombosis) may form in your dialysis shunt. This is more likely if you have low blood pressure or if your fistula has complications.
• Blood clots may also form in your haemodialysis system. Your doctor may decide to increase your heparin dose during dialysis.
Tell your doctor or nurse immediately if you are aware of any of these effects, or if you notice any other effects while you are receiving treatment with Eporise®.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Eporise®
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date, which is stated on the box and on the label after the letters EXP. The expiry date refers to the last day of that month.
Store in a refrigerator (20C-80C). You may take Eporise® out of the refrigerator and keep it at room temperature (up to 250C) for no longer than 3 days. Once a syringe has been removed from the refrigerator and has reached room temperature (up to 250C) it must either be used within 3 days or disposed of.
Do not freeze or shake.
Store in the original package in order to protect it from light.
Do not use this medicine if you notice that the seal is broken or if the liquid is coloured or you can see particles floating in it. In the event of either being observed, discard the medicinal product.
Do not throw away any medicines via waste water or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment
6. Contents of the pack and other information
What Eporise® tablets contain
The active substances of Eporise® are erythropoietin.
Eporise® is formulated as a sterile clear solution for intravenous or subcutaneous administration.
Each pre-filled syringe of Eporise 2000/ 4000/ 10000/20000 contains Erythropoietin Concentrated Solution IP 2000/ 4000/ 10000/20000 IU respectively, Albumin (Human) 2.5mg, Sodium Citrate 5.8mg, Sodium Chloride 5.8mg and citric acid 0.06mg in water for injection IP (pH 6.9±0.3).
This formulation contains no preservatives.
Each pre-filled syringe of Eporise 40,000 contains Erythropoietin Concentrated Solution IP 40,000 IU, Albumin (Human) 2.5mg, Sodium Phosphate Monobasic monohydrate 1.2mg, Sodium Phosphate Dibasic 1.8mg, Sodium Citrate 0.7 mg, Sodium Chloride 5.8mg and citric acid 6.8 mcg in water for injection IP, (pH 6.9±0.3).
This formulation contains no preservatives.
Presentation/pack size
Pre-filled syringes of 2000/ 4000/ 10000/20000/ 40000 International units