Eslo 5 AT Tablets
Therapy Area
Cardiology
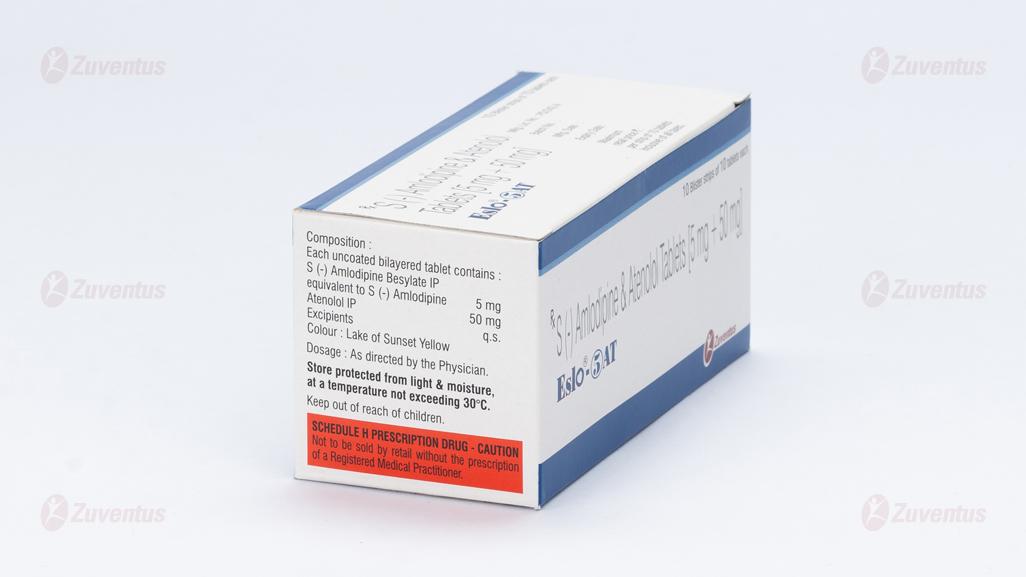
Composition
Each uncoated bilayered tablet contains :
S (-) Amlodipine Besylate IP
equivalent to S (-) Amlodipine 5 mg
Atenolol IP 50 mg
Excipients q.s.
Colour : Lake of Sunset Yellow
THERAPEUTIC CATEGORY :
Antihypertensive, antianginal agent
Description
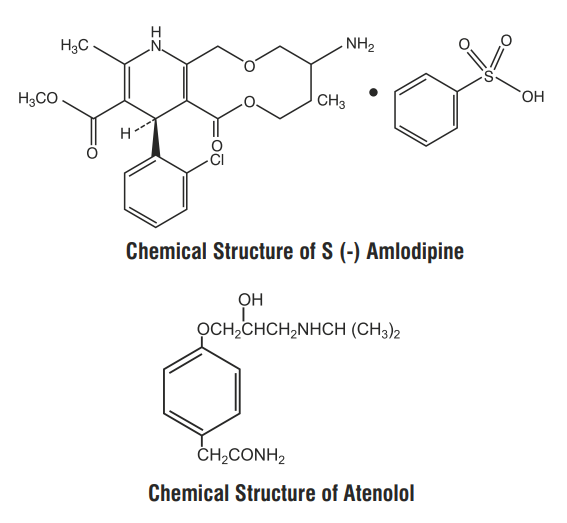
S (-) Amlodipine, the chirally pure form of Amlodipine is a calcium channel antagonist belonging to the dihydropyridine class. The chemical name of S (-) Amlodipine is (S)-3-ethyl5-1-methyl-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)- 1,4-dih-ydro-6-methyl-3,5-pyridinedicarboxylate. Its empirical l formula is C20H25ClN2O5C6H6O3 S,with a molecular 20 25 2 5 6 6 3 weight of 567.1
Atenolol is a β1-selective receptor antagonist and produces the antihypertensive activity. It is chemically described as benzeneacetamide, 4-[2'-hydroxy-3'-[(1-methylethyl) amino] propoxy]. Atenolol has a molecular weight of 266.34 with molecular formula C14H 22N2O3 .
Clinical Pharmacology :
S (-) Amlodipine, the chirally pure form of Amlodipine is a calcium channel antagonist belonging to the dihydropyridine class. The S (-) isomer of Amlodipine is found to possess greater pharmacological effects than R (+) Amlodipine. S (-) Amlodipine is 1000 times more potent than the R (+) isomer in binding to the dihydropyridine receptor. In humans, the dominant effects of Amlodipine are consequent to vasodilation. S (-) Amlodipine lowers peripheral vascular resistance without causing a reflex tachycardia. In patients with angina pectoris S (-) Amlodipine reduces the myocardial oxygen demand by causing a direct vasodilation of the coronary artery and arterioles. Atenolol is a β1-selective receptor antagonist and produces the antihypertensive activity. β-adrenergic antagonists slow the heart rate and decrease myocardial contractility. These agents decrease the effect of catecholamines on the determinants of myocardial oxygen consumption thus improving the relationship between cardiac oxygen supply and demand. Combinations of Amlodipine and Atenolol have been used successfully to treat hypertension and angina. Since S (-) Amlodipine is the chirally pure form of Amlodipine, the combination of S (-) Amlodipine and Atenolol is highly beneficial in the treatment of hypertension and angina.
Pharmacokinetics:
Administration of S (-) Amlodipine 2.5 mg as a single dose in the fasting state produced maximum plasma concentration (Cmax) of 8.30 ± 1.071 ng/ ml in 2.73 ± 0.88 hrs. (Tmax). Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. Ex vivo studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. The mean AUC0-t value (t= 48 hrs.) of Tablet S (-) Amlodipine (2.5 mg) is 95.33 ± 14.45 ng.hr/ml. The AUC0-∞ value is recorded to be 140.91± 28.06 ng.hr/ml. The plasma elimination half life of S (-) Amlodipine has been found to be 31.09 ± 12.65 hrs.
Approximately 50% of an oral dose of Atenolol is absorbed from the gastro-intestinal tract and the remainder being excreted unchanged in the faeces. Maximum plasma concentrations are reached within two to four hours after a single oral dose and doses of 50 mg and 100 mg produce mean peak plasma concentrations of approximately 300 and 700 ng/mL, respectively. The plasma half-life (t½) is 6-7 hours and Atenolol is effective for at least 24 hours after a single oral dose. Atenolol is 6-16% plasma protein bound and is extensively distributed to extravascular tissues, but only a small amount is found in the central nervous system. Approximately 10% of Atenolol is metabolised in man. Approximately 47% and 53% of an oral dose is eliminated in the urine and faeces respectively.
Indications
For treatment of Essential Hypertension & Angina Pectoris.
Contraindications
Hypersensitivity to any of the components of the formulation, sinus bradycardia, second and higher degrees of heart block, cardiogenic shock, hypotension and overt cardiac failure.
Dosage and Administration
One tablet to be administered once daily.
Adverse Reactions
Gingival hypertrophy and alopecia has been seen with S (-) Amlodipine. On the basis of the clinical data available, only minor side effects have been reported with the use of S (-) Amlodipine. On the basis of clinical data available, adverse events reported in less than 5% of the patients included anxiety (0.43%), anorexia (0.43%), irritation (0.43%), headache (2.13%) and facial flushing (2.13%). Caution should be exercised when administering S (-) Amlodipine to patients with impaired hepatic and renal function.
Common adverse events seen with Atenolol are as follows : Sleep disturbances, purpura, thrombocytopenia, leucopenia, mood changes, depression, anxiety, nightmares, confusion, psychosis, hallucinations, dizziness, headache, paraesthesia of extremities, dry eyes, impaired vision, visual disturbances, bradycardia, heart failure deterioration, precipitation of heart block, cold extremities, postural hypotension which may be associated with syncope, bronchospasmm, gastrointestinal disturbances, constipation, dry mouth, elevations of transaminase levels, hepatic toxicity including intrahepatic cholestasis, alopecia, psoriasiform skin reactions, exacerbation of psoriasis, skin rashes, impotence, fatigue, sweating.
Drug Interactions
Clinical studies have shown that S (-) Amlodipine when combined with aspirin, nitrates, beta-blockers, statins, ACE inhibitors, H blockers, and Proton Pump Inhibitors produced no drug interactions. No drug interaction has been reported 2 with the use of Atenolol.
Warnings
Increased Angina and/or Myocardial Infarction
Rarely, patients, particularly those with severe obstructive coronary artery disease, have developed documented increased frequency, duration and/or severity of angina or acute myocardial infarction on starting calcium channel blocker therapy or at the time of dosage increase. The mechanism of this effect has not been elucidated.
Cardiac Failure
Sympathetic stimulation is necessary in supporting circulatory function in congestive heart failure, and beta blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe failure. In patients who have congestive heart failure controlled by digitalis and/or diuretics, Atenolol should be administered cautiously. Both digitalis and Atenolol slow AV conduction. In general, calcium channel blockers should also be used with caution in patients with heart failure.
Bronchospastic Diseases
Patients with bronchospastic disease should, in general, not receive beta blockers. Because of its relative β1 selectivity, however, Atenolol may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment.
Thyrotoxicosis
Beta-adrenergic blockade may mask certain clinical signs (eg, tachycardia) of hyperthyroidism. Abrupt withdrawal of beta blockade might precipitate a thyroid storm; therefore, patients suspected of developing thyrotoxicosis from which Atenolol therapy is to be withdrawn and should be monitored closely.
Untreated Pheochromocytoma
Atenolol should not be given to patients with untreated pheochromocytoma
Pregnancy and Fetal Injury
There is no data available on the use of S (-) Amlodipine in pregnant and lactating women. Atenolol can cause fetal harm when administered to a pregnant woman. Atenolol crosses the placental barrier and appears in cord blood. Administration of Atenolol, starting in the second trimester of pregnancy, has been associated with the birth of infants that are small for gestational age. No studies have been performed on the use of Atenolol in the first trimester and the possibility of fetal injury cannot be excluded. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Neonates born to mothers who are receiving Atenolol at parturition or breast-feeding may be at risk for hypoglycemia and bradycardia. Caution should be exercised when Atenolol is administered during pregnancy or to a woman who is breast-feeding.
Precautions :
Hepatic impairment and renal impairment
No controlled clinical study of S (-) Amlodipine has been performed in patients with hepatic impairment and renal impairment. Clinical studies in patients with normal liver function have shown that there is no elevation in the hepatic enzymes with the use of S (-) Amlodipine. Since about 90% of the drug is excreted unchanged in urine, no dosage adjustment is required in patients with hepatic impairment. There is no significant hepatic metabolism of Atenolol and more than 90% of that absorbed reaches the systemic circulation unaltered. Plasma half-life is raised in patients with renal failure with S (-) Amlodipine. Since Atenolol is excreted via the kidneys, the dosage should be adjusted in cases of severe impairment of renal function. Hence, caution should be taken while administering the combination to such patients.
Children
Safety and effectiveness of this product in children has not been established.
Overdosage
Overdosage with racemic Amlodipine and Atenolol may cause excessive peripheral vasodilation with marked hypotension possibly a reflex tachycardia, acute cardiac insufficiency and bronchospasm. Hence, caution should be taken in case of an overdosage with Eslo-5AT tablet. If massive overdose occurs, active cardiac and respiratory monitoring should be instituted. Frequent blood pressure measurements should be performed. Should hypotension occur, cardiovascular support including elevation of the extremities and the judicious administration of fluids should be initiated. If hypotension remains unresponsive to these conservative measures, administration of vasopressors (such as phenylephrine) should be considered with attention to circulating volume and urine output. If massive overdose occurs, gastric lavage should be employed. As this product is highly plasma protein bound, hemodialysis is not likely to be of benefit.
Storage
Store protected from light & moisture, at a temperature not exceeding 30°C.
Keep out of reach of children.
Shelf-life
Refer on the pack.
Presentation
A blister strip of 10 tablets.
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you:
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Eslo®-AT and Eslo®5-AT is and what it is used for
2. What you need to know before you take Eslo®-AT and Eslo®5-AT
3. How to take Eslo®-AT and Eslo®5-AT
4. Possible side effects
5. How to store Eslo®-AT and Eslo®5-AT
6. Contents of the pack and other information
1. What Eslo®-AT and Eslo®5-AT is and what it is used for
Eslo®-AT and Eslo®5-AT tablets contain two substances called S(-)Amlodipine and Atenolol. Both of these substances help to control high blood pressure.
− S(-)Amlodipine belongs to a group of substances called “calcium channel blockers”. S(-)Amlodipine stops calcium from moving into the blood vessel wall which stops the blood vessels from tightening.
− Atenolol belongs to a group of substances called “beta-blockers”. Beta-blockers slow the heartbeat, lessen the force with which the heart muscle contracts and reduce blood vessel contraction in the heart, brain, and throughout the body.
This means that both substances help stop the blood vessel tightening and prevent chest pain. As a result, the blood vessels relax and blood pressure is lowered.
Eslo®-AT and Eslo®5-AT are used to treat high blood pressure and angina.
2. What you need to know before you take Eslo®-AT and Eslo®5-AT
Do not take Eslo®-AT and Eslo®5-AT:
- if you are allergic to S(-)Amlodipine, amlodipine, atenolol, any drug of a related class, or any of the other ingredients of this medicine (listed in section 6).
- if you have severe liver problems if you are pregnant
- If you have ever had any of the following heart problems: -heart failure which is not under control (this usually makes you breathless and causes your ankles to swell) - second- or third-degree heart block (a condition which may be treated by a pacemaker) - very slow or very uneven heartbeats, very low blood pressure or very poor circulation.
- If you have been told that you have higher than normal levels of acid in your blood (metabolic acidosis)
If any of the above applies to you, do not take Eslo®-AT and Eslo®5-AT and talk to your doctor.
Warnings and precautions
Talk to your doctor before taking Eslo®-AT and Eslo®5-AT Tablets:
- You have asthma, wheezing or any other similar breathing problems, or you get allergic reactions.
- If you have ever had asthma or wheezing, do not take this medicine without first checking with your doctor
- if you have been sick (vomiting or diarrhoea). You have poor blood circulation or controlled heart failure.
- You have first-degree heart block You have diabetes.
- Your medicine may change how you respond to having low blood sugar.
- You may feel your heart beating faster. You have thyrotoxicosis (a condition caused by an overactive thyroid gland).
- Your medicine may hide the symptoms of thyrotoxicosis.
- You have problems with your kidneys. You may need to have some check-ups during your treatment.
- you have been told that you have high blood pressure due to a tumour near your kidney (phaeochromocytoma),
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before taking Eslo®-AT and Eslo®5-AT.
Children and adolescents
The use of Eslo®-AT and Eslo®5-AT in children and adolescents is not recommended (aged below 18 years old).
Other medicines and Eslo®-AT and Eslo®5-AT Tablets
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Your doctor may need to change your dose and/or take other precautions. In some cases, you may have to stop taking one of the medicines. This applies especially to the medicines listed below:
In particular, tell your doctor if you are taking any of the following medicines:
anti-fungal medicines (e.g. ketoconazole, itraconazole)
protease inhibitors used to treat HIV (e.g. ritonavir, indinavir, nelfinavir)
rifampicin, erythromycin, clarithromycin (antibiotics)
hypericum perforatum (St. John’s wort)
verapamil, diltiazem (heart medicines)
dantrolene (infusion for severe body temperature abnormalities)
simvastatin (a medicine to reduce blood cholesterol levels)
tacrolimus, sirolimus, temsirolimus and everolimus (medicines used to change the way your immune system works).
ciclosporin (if you have had a kidney transplant and are taking ciclosporin)
as amlodipine may alter the amount of this medicine in your blood. verapamil,
diltiazem and nifedipine (for high blood pressure or chest pain).
clonidine (for high blood pressure or migraine).
If you are taking clonidine, do not stop taking clonidine unless your doctor tells you to do so.
If you have to stop taking clonidine, your doctor will give you careful instructions about how to do it.
digoxin (for heart problems).
disopyramide, quinidine or amiodarone (for an uneven heart beat).
adrenaline, also known as epinephrine (a medicine that stimulate the heart)
ibuprofen or indometacin (for pain and inflammation) insulin or medicines
that you take by mouth for diabetes medicines to treat
nose or sinus congestion or other cold remedies (including those you can buy in pharmacy).
Eslo®-AT and Eslo®5-AT Tablets with food and drink
Grapefruit juice and grapefruit should not be consumed by people who are taking Eslo®-AT and Eslo®5-AT Tablets. This is because grapefruit and grapefruit juice can lead to an increase in the blood levels of the active ingredient S(-)Amlodipine, which can cause an unpredictable increase in the blood pressure-lowering effect of S(-)Amlodipine.
Pregnancy, breast-feeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Your medicine is not likely to affect you being able to drive or use any tools or machines. However, it is best to wait to see how your medicine affects you before trying these activities. If you feel dizzy or tired when taking this medicine, do not drive or use any tools or machines.
3. How to take Eslo®-AT and Eslo®5-AT Tablets
Always take or give this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. This will help you get the best results and lower the risk of side effects.
The usual dose of Eslo®-AT and Eslo®5-AT is one tablet per day.
− It is preferable to take your medicine at the same time each day.
− Swallow the tablets with a glass of water.
If you take more Eslo®-AT and Eslo®5-AT than you should
If you have taken too many tablets of Eslo®-AT and Eslo®5-AT, consult a doctor immediately. Take the medicine pack with you so that the tablets can be identified.
If you forget to take or give Eslo®-AT and Eslo®5-AT
If you forget to take this medicine, take it as soon as you remember. Then take your next dose at its usual time. However, if it is almost time for your next dose, skip the dose you missed. Do not take a double dose to make up for a forgotten tablet.
If you stop taking Eslo®-AT and Eslo®5-AT
Stopping your treatment with Eslo®-AT and Eslo®5-AT may cause your disease to get worse. Do not stop taking your medicine unless your doctor tells you to.
4. Possible side effects
This medicine can cause side effects like all medicines, although not everybody gets them.
Some side effects can be serious and need immediate medical attention: A few patients have experienced these serious side effects (may affect up to 1 in 1,000 people). If any of the following happens, tell your doctor straight away: Allergic reaction with symptoms such as rash, itching, swelling of face or lips or tongue, difficulty breathing, low blood pressure (feeling of faintness, light-headedness).
Other possible side effects of Eslo®-AT and Eslo®5-AT:
Common (may affect up to 1 in 10 people): Headache, dizziness, sleepiness, (especially at the beginning of treatment), palpitations (awareness of your heartbeat), flushing, abdominal pain, feeling sick (nausea), altered bowel habits which includes diarrhoea and constipation, indigestion, tiredness, unusual weakness, visual disturbances including double vision, shortness of breath, muscle cramps, cold hands and feet, diarrhoea, feeling sick (nausea), feeling tired
Uncommon (may affect up to 1 in 100 people): Mood changes (including feeling anxious), feeling depressed, sleeplessness, trembling, taste abnormalities, fainting, numbness or tingling sensation in your limbs; decreased sensation, tinging in the ears, decreased heart rate, low blood pressure, sneezing/running nose caused by inflammation of the lining of the nose (rhinitis), cough, dry mouth, vomiting (being sick), hair-loss, increased sweating, itchy skin, red patches on skin, skin discolouration, rash and hives, purple patches on the skin, disorder in passing urine, increased need to urinate at night, increased number of times of passing urine, inability to obtain an erection; discomfort or enlargement of the breasts in men, pain, chest pain, feeling unwell, joint or muscle pain, back pain, weight increase or decrease, disturbed sleep
Rare (may affect up to 1 in 1,000 people): Heart block (which can cause dizziness, abnormal heartbeat, tiredness or fainting), numbness or spasm in your fingers which is followed by warmth and pain (Raynaud’s disease), nightmares, feeling confused, changes in personality (psychoses) or seeing or hearing things that are not there (hallucinations), dizziness (particularly when standing up), tingling of your hands, thinning of your hair, disturbances of vision, skin rash, purple marks on your skin, being unable to get an erection (impotence), yellowing of your skin or the whites of your eyes (jaundice), reduced numbers of platelets in your blood (this may make you bruise more easily).
If any of these affect you severely, tell your doctor.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Eslo®-AT and Eslo®5-AT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister.
Do not store above 30°C.
Store in the original package in order to protect it from moisture.
Do not use any Eslo®-AT and Eslo®5-AT pack that is damaged or shows signs of tampering.
6. Contents of the pack and other information
What Eslo®-AT and Eslo®5-AT tablets contain
Eslo®-AT tablets
The active substances of Eslo®-AT are S(-)Amlodipine as s(-) Amlodipine besylate and Atenolol. Each tablet contains 2.5 mg S(-)Amlodipine and 50 mg Atenolol.
Eslo®5-AT tablets
The active substances of Eslo®5-AT are S(-)Amlodipine as s(-) Amlodipine besylate and Atenolol. Each tablet contains 5 mg S(-)Amlodipine and 50 mg Atenolol.
What Eslo®-AT and Eslo®5-AT looks like and contents of the pack
Eslo®-AT is available in packs of 15 tablets per strip.
Eslo®5-AT is available in packs of 10 tablets per strip.