Etospeed-90 Tablets
Therapy Area
Pain management
1.0 Generic Name
Etoricoxib film coated tablets
2.0 Qualitative and quantitative composition
ETOSPEEDTM- 90
Each film coated tablet contains:
Etoricoxib 90 mg
3.0 Dosage form and strength
Tablets, 90 mg
4.0 Clinical particulars
Therapeutic indication
ETOSPEED is indicated in adults and adolescents 16 years of age and older for the symptomatic relief of osteoarthritis (OA), rheumatoid arthritis (RA), ankylosing spondylitis, and the pain and signs of inflammation associated with acute gouty arthritis. ETOSPEED is indicated in adults and adolescents 16 years of age and older for the short-term treatment of moderate pain associated with dental surgery. The decision to prescribe a selective COX-2 inhibitor should be based on an assessment of the individual patient's overall risks.
Posology and method of administration
Posology
As the cardiovascular risks of etoricoxib may increase with dose and duration of exposure, the shortest duration possible and the lowest effective daily dose should be used. The patient's need for symptomatic relief and response to therapy should be re-evaluated periodically, especially in patients with osteoarthritis.
Osteoarthritis
The recommended dose is 30 mg once daily. In some patients with insufficient relief from symptoms, an increased dose of 60 mg once daily may increase efficacy. In the absence of an increase in therapeutic benefit, other therapeutic options should be considered.
Rheumatoid arthritis
The recommended dose is 60 mg once daily. In some patients with insufficient relief from symptoms, an increased dose of 90 mg once daily may increase efficacy. Once the patient is clinically stabilised, down-titration to a 60 mg once daily dose may be appropriate. In the absence of an increase in therapeutic benefit, other therapeutic options should be considered.
Ankylosing spondylitis
The recommended dose is 60 mg once daily. In some patients with insufficient relief from symptoms, an increased dose of 90 mg once daily may increase efficacy. Once the patient is clinically stabilised, down-titration to a 60 mg once daily dose may be appropriate. In the absence of an increase in therapeutic benefit, other therapeutic options should be considered.
Acute pain conditions
For acute pain conditions, etoricoxib should be used only for the acute symptomatic period.
Acute gouty arthritis
The recommended dose is 120 mg once daily. In clinical trials for acute gouty arthritis, etoricoxib was given for 8 days.
Postoperative dental surgery pain
The recommended dose is 90 mg once daily, limited to a maximum of 3 days. Some patients may require other postoperative analgesia in addition to ETOSPEED during the three-day treatment period. Doses greater than those recommended for each indication have either not demonstrated additional efficacy or have not been studied. Therefore:
The dose for OA should not exceed 60 mg daily.
The dose for RA and ankylosing spondylitis should not exceed 90 mg daily.
The dose for acute gout should not exceed 120 mg daily, limited to a maximum of 8 days’ treatment.
The dose for postoperative acute dental surgery pain should not exceed 90 mg daily, limited to a maximum of 3 days.
Special populations
Elderly patients
No dosage adjustment is necessary for elderly patients. As with other drugs, caution should be exercised in elderly patients.
Patients with hepatic impairment
Regardless of indication, in patients with mild hepatic dysfunction (Child-Pugh score 5-6) a dose of 60 mg once daily should not be exceeded. In patients with moderate hepatic dysfunction (Child-Pugh score 7-9), regardless of indication, the dose of 30 mg once daily should not be exceeded. Clinical experience is limited particularly in patients with moderate hepatic dysfunction and caution is advised. There is no clinical experience in patients with severe hepatic dysfunction (Child-Pugh score ≥10); therefore, its use is contraindicated in these patients.
Patients with renal impairment
No dosage adjustment is necessary for patients with creatinine clearance ≥ 30 mL/min. The use of etoricoxib in patients with creatinine clearance < 30 mL/min is contra-indicated.
Paediatric population
Etoricoxib is contra-indicated in children and adolescents under 16 years of age.
Method of administration
ETOSPEED is administered orally and may be taken with or without food. The onset of the effect of the medicinal product may be faster when ETOSPEED is administered without food. This should be considered when rapid symptomatic relief is needed.
4.3 Contraindications
• Hypersensitivity to the active substance or to any of the excipients.
• Active peptic ulceration or active gastro-intestinal (GI) bleeding.
• Patients who, after taking acetylsalicylic acid or NSAIDs including COX-2 (cyclooxygenase-2) inhibitors, experience bronchospasm, acute rhinitis, nasal polyps, angioneurotic oedema, urticaria, or allergic-type reactions.
• Pregnancy and lactation.
• Severe hepatic dysfunction (serum albumin < 25 g/L or Child-Pugh score ≥10).
• Estimated renal creatinine clearance < 30 mL/min.
• Children and adolescents under 16 years of age.
• Inflammatory bowel disease.
• Congestive heart failure (NYHA II-IV).
• Patients with hypertension whose blood pressure is persistently elevated above 140/90 mmHg and has not been adequately controlled.
• Established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease.
4.4 Special warnings and precautions for use
Gastrointestinal effects
Upper gastrointestinal complications [perforations, ulcers or bleedings (PUBs)], some of them resulting in fatal outcome, have occurred in patients treated with etoricoxib. Caution is advised with treatment of patients most at risk of developing a gastrointestinal complication with NSAIDs; the elderly, patients using any other NSAID or acetylsalicylic acid concomitantly or patients with a prior history of gastrointestinal disease, such as ulceration and GI bleeding.
There is a further increase in the risk of gastrointestinal adverse effects (gastrointestinal ulceration or other gastrointestinal complications) when etoricoxib is taken concomitantly with acetylsalicylic acid (even at low doses). A significant difference in GI safety between selective COX-2 inhibitors + acetylsalicylic acid vs. NSAIDs + acetylsalicylic acid has not been demonstrated in long-term clinical trials.
Cardiovascular effects
Clinical trials suggest that the selective COX-2 inhibitor class of drugs may be associated with a risk of thrombotic events (especially myocardial infarction (MI) and stroke), relative to placebo and some NSAIDs. As the cardiovascular risks of etoricoxib may increase with dose and duration of exposure, the shortest duration possible and the lowest effective daily dose should be used. The patient's need for symptomatic relief and response to therapy should be re-evaluated periodically, especially in patients with osteoarthritis. Patients with significant risk factors for cardiovascular events (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking) should only be treated with etoricoxib after careful consideration.
COX-2 selective inhibitors are not a substitute for acetylsalicylic acid for prophylaxis of cardiovascular thromboembolic diseases because of their lack of antiplatelet effect. Therefore, antiplatelet therapies should not be discontinued.
Renal effects
Renal prostaglandins may play a compensatory role in the maintenance of renal perfusion. Therefore, under conditions of compromised renal perfusion, administration of etoricoxib may cause a reduction in prostaglandin formation and, secondarily, in renal blood flow, and thereby impair renal function. Patients at greatest risk of this response are those with pre-existing significantly impaired renal function, uncompensated heart failure, or cirrhosis. Monitoring of renal function in such patients should be considered.
Fluid retention, oedema and hypertension
As with other medicinal products known to inhibit prostaglandin synthesis, fluid retention, oedema and hypertension have been observed in patients taking etoricoxib. All Nonsteroidal Anti-inflammatory Drugs (NSAIDs), including etoricoxib, can be associated with new onset or recurrent congestive heart failure. For information regarding a dose related response for etoricoxib. Caution should be exercised in patients with a history of cardiac failure, left ventricular dysfunction, or hypertension and in patients with pre-existing oedema from any other reason. If there is clinical evidence of deterioration in the condition of these patients, appropriate measures including discontinuation of etoricoxib should be taken.
Etoricoxib may be associated with more frequent and severe hypertension than some other NSAIDs and selective COX2 inhibitors, particularly at high doses. Therefore, hypertension should be controlled before treatment with etoricoxib and special attention should be paid to blood pressure monitoring during treatment with etoricoxib. Blood pressure should be monitored within two weeks after initiation of treatment and periodically thereafter. If blood pressure rises significantly, alternative treatment should be considered.
Hepatic effects
Elevations of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) (approximately three or more times the upper limit of normal) have been reported in approximately 1 % of patients in clinical trials treated for up to one year with etoricoxib 30, 60 and 90 mg daily.
Any patients with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver function test has occurred, should be monitored. If signs of hepatic insufficiency occur, or if persistently abnormal liver function tests (three times the upper limit of normal) are detected, etoricoxib should be discontinued.
General
If during treatment, patients deteriorate in any of the organ system functions described above, appropriate measures should be taken and discontinuation of etoricoxib therapy should be considered. Medically appropriate supervision should be maintained when using etoricoxib in the elderly and in patients with renal, hepatic, or cardiac dysfunction.
Caution should be used when initiating treatment with etoricoxib in patients with dehydration. It is advisable to rehydrate patients prior to starting therapy with etoricoxib.
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs and some selective COX-2 inhibitors during post-marketing surveillance. Patients appear to be at highest risk for these reactions early in the course of therapy with the onset of the reaction occurring in the majority of cases within the first month of treatment. Serious hypersensitivity reactions (such as anaphylaxis and angioedema) have been reported in patients receiving etoricoxib. Some selective COX-2 inhibitors have been associated with an increased risk of skin reactions in patients with a history of any drug allergy. Etoricoxib should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
Etoricoxib may mask fever and other signs of inflammation.
Caution should be exercised when co-administering etoricoxib with warfarin or other oral anticoagulants.
The use of etoricoxib, as with any medicinal product known to inhibit cyclooxygenase / prostaglandin synthesis, is not recommended in women attempting to conceive.
4.5 Interaction with other medicinal products and other forms of interaction
Pharmacodynamics interactions
Oral anticoagulants: In subjects stabilised on chronic warfarin therapy, the administration of etoricoxib 120 mg daily was associated with an approximate 13 % increase in prothrombin time International Normalised Ratio (INR). Therefore, patients receiving oral anticoagulants should be closely monitored for their prothrombin time INR, particularly in the first few days when therapy with etoricoxib is initiated or the dose of etoricoxib is changed.
Diuretics, ACE inhibitors and Angiotensin II Antagonists: NSAIDs may reduce the effect of diuretics and other antihypertensive drugs. In some patients with compromised renal function (e.g. dehydrated patients or elderly patients with compromised renal function) the co-administration of an ACE inhibitor or Angiotensin II antagonist and agents that inhibit cyclo-oxygenase may result in further deterioration of renal function, including possible acute renal failure, which is usually reversible. These interactions should be considered in patients taking etoricoxib concomitantly with ACE inhibitors or angiotensin II antagonists. Therefore, the combination should be administered with caution, especially in the elderly. Patients should be adequately hydrated and consideration should be given to monitoring of renal function after initiation of concomitant therapy, and periodically thereafter.
Acetylsalicylic Acid: In a study in healthy subjects, at steady state, etoricoxib 120 mg once daily had no effect on the anti-platelet activity of acetylsalicylic acid (81 mg once daily). Etoricoxib can be used concomitantly with acetylsalicylic acid at doses used for cardiovascular prophylaxis (low-dose acetylsalicylic acid). However, concomitant administration of low dose acetylsalicylic acid with etoricoxib may result in an increased rate of GI ulceration or other complications compared to use of etoricoxib alone. Concomitant administration of etoricoxib with doses of acetylsalicylic acid above those for cardiovascular prophylaxis or with other NSAIDs is not recommended.
Cyclosporin and tacrolimus: Although this interaction has not been studied with etoricoxib, coadministration of cyclosporin or tacrolimus with any NSAID may increase the nephrotoxic effect of cyclosporin or tacrolimus. Renal function should be monitored when etoricoxib and either of these drugs is used in combination.
Pharmacokinetic interactions
The effect of etoricoxib on the pharmacokinetics of other drugs
Lithium: NSAIDs decrease lithium renal excretion and therefore increase lithium plasma levels. If necessary, monitor blood lithium closely and adjust the lithium dosage while the combination is being taken and when the NSAID is withdrawn.
Methotrexate: Two studies investigated the effects of etoricoxib 60, 90 or 120 mg administered once daily for seven days in patients receiving once-weekly methotrexate doses of 7.5 to 20 mg for rheumatoid arthritis. Etoricoxib at 60 and 90 mg had no effect on methotrexate plasma concentrations or renal clearance. In one study, etoricoxib 120 mg had no effect, but in the other study, etoricoxib 120 mg increased methotrexate plasma concentrations by 28 % and reduced renal clearance of methotrexate by 13 %. Adequate monitoring for methotrexate-related toxicity is recommended when etoricoxib and methotrexate are administered concomitantly.
Oral contraceptives: Etoricoxib 60 mg given concomitantly with an oral contraceptive containing 35 micrograms ethinyl estradiol (EE) and 0.5 to 1 mg norethindrone for 21 days increased the steady state AUC0-24hr of EE by 37 %. Etoricoxib 120 mg given with the same oral contraceptive concomitantly or separated by 12 hours, increased the steady state
AUC0-24hr of EE by 50 to 60 %. This increase in EE concentration should be considered when selecting an oral contraceptive for use with etoricoxib. An increase in EE exposure can increase the incidence of adverse events associated with oral contraceptives (e.g., venous thrombo-embolic events in women at risk).
Hormone Replacement Therapy (HRT): Administration of etoricoxib 120 mg with hormone replacement therapy consisting of conjugated estrogens (0.625 mg premarin) for 28 days, increased the mean steady state AUC0-24hr of unconjugated estrone (41 %), equilin (76 %), and 17-β-estradiol (22 %). The effect of the recommended chronic doses of etoricoxib (30, 60, and 90 mg) has not been studied. The effects of etoricoxib 120 mg on the exposure (AUC0-24hr) to these estrogenic components of PREMARIN were less than half of those observed when PREMARIN was administered alone and the dose was increased from 0.625 to 1.25 mg. The clinical significance of these increases is unknown, and higher doses of PREMARIN were not studied in combination with etoricoxib. These increases in estrogenic concentration should be taken into consideration when selecting post-menopausal hormone therapy for use with etoricoxib because the increase in oestrogen exposure might increase the risk of adverse events associated with HRT.
Prednisone/prednisolone: In drug-interaction studies, etoricoxib did not have clinically important effects on the pharmacokinetics of prednisone/prednisolone.
Digoxin: Etoricoxib 120 mg administered once daily for 10 days to healthy volunteers did not alter the steady-state plasma AUC0-24hr or renal elimination of digoxin. There was an increase in digoxin Cmax (approximately 33 %). This increase is not generally important for most patients. However, patients at high risk of digoxin toxicity should be monitored for this when etoricoxib and digoxin are administered concomitantly.
Effect of etoricoxib on drugs metabolised by sulfotransferases
Etoricoxib is an inhibitor of human sulfotransferase activity, particularly SULT1E1, and has been shown to increase the serum concentrations of ethinyl estradiol. While knowledge about effects of multiple sulfotransferases is presently limited and the clinical consequences for many drugs are still being examined, it may be prudent to exercise care when administering etoricoxib concurrently with other drugs primarily metabolised by human sulfotransferases (e.g., oral salbutamol and minoxidil).
Effect of etoricoxib on drugs metabolised by CYP isoenzymes
Based on in vitro studies, etoricoxib is not expected to inhibit cytochromes P450 (CYP) 1A2, 2C9, 2C19, 2D6, 2E1 or 3A4. In a study in healthy subjects, daily administration of etoricoxib 120 mg did not alter hepatic CYP3A4 activity as assessed by the erythromycin breath test.
Effects of other drugs on the pharmacokinetics of etoricoxib
The main pathway of etoricoxib metabolism is dependent on CYP enzymes. CYP3A4 appears to contribute to the metabolism of etoricoxib in vivo. In vitro studies indicate that CYP2D6, CYP2C9, CYP1A2 and CYP2C19 also can catalyse the main metabolic pathway, but their quantitative roles have not been studied in vivo.
Ketoconazole: Ketoconazole, a potent inhibitor of CYP3A4, dosed at 400 mg once a day for 11 days to healthy volunteers, did not have any clinically important effect on the single-dose pharmacokinetics of 60 mg etoricoxib (43 % increase in AUC).
Voriconazole and Miconazole: Co-administration of either oral voriconazole or topical miconazole oral gel, strong CYP3A4 inhibitors, with etoricoxib caused a slight increase in exposure to etoricoxib, but is not considered to be clinically meaningful based on published data.
Rifampicin: Co-administration of etoricoxib with rifampicin, a potent inducer of CYP enzymes, produced a 65 % decrease in etoricoxib plasma concentrations. This interaction may result in recurrence of symptoms when etoricoxib is co-administered with rifampicin. While this information may suggest an increase in dose, doses of etoricoxib greater than those listed for each indication have not been studied in combination with rifampicin and are therefore not recommended.
Antacids: Antacids do not affect the pharmacokinetics of etoricoxib to a clinically relevant extent.
4.6 Fertility, Pregnancy and Lactation
Pregnancy
No clinical data on exposed pregnancies are available for etoricoxib. Studies in animals have shown reproductive toxicity. The potential for human risk in pregnancy is unknown. Etoricoxib, as with other medicinal products inhibiting prostaglandin synthesis, may cause uterine inertia and premature closure of the ductus arteriosus during the last trimester. Etoricoxib is contraindicated in pregnancy. If a woman becomes pregnant during treatment, etoricoxib must be discontinued.
Breast-feeding
It is not known whether etoricoxib is excreted in human milk. Etoricoxib is excreted in the milk of lactating rats. Women who use etoricoxib must not breast feed.
Fertility
The use of etoricoxib, as with any drug substance known to inhibit COX-2, is not recommended in women attempting to conceive.
4.7 Effects on ability to drive and use machines
Patients who experience dizziness, vertigo or somnolence while taking etoricoxib should refrain from driving or operating machinery.
4.8 Undesirable effects
Summary of the safety profile
In clinical trials, etoricoxib was evaluated for safety in 9,295 individuals, including 6,757 patients with OA, RA, chronic low back pain or ankylosing spondylitis (approximately 600 patients with OA or RA were treated for one year or longer).
In clinical studies, the undesirable effects profile was similar in patients with OA or RA treated with etoricoxib for one year or longer.
In a clinical study for acute gouty arthritis, patients were treated with etoricoxib 120 mg once daily for eight days. The adverse experience profile in this study was generally similar to that reported in the combined OA, RA, and chronic low back pain studies.
In a cardiovascular safety outcomes programme of pooled data from three active comparator-controlled trials, 17, 412 patients with OA or RA were treated with etoricoxib (60 mg or 90 mg) for a mean duration of approximately 18 months. The safety data and details from this programme.
In clinical studies for acute postoperative dental pain following surgery including 614 patients treated with etoricoxib (90 mg or 120 mg), the adverse experience profile in these studies was generally similar to that reported in the combined OA, RA, and chronic low back pain studies.
Tabulated list of adverse reactions
The following undesirable effects were reported at an incidence greater than placebo in clinical trials in patients with OA, RA, chronic low back pain or ankylosing spondylitis treated with etoricoxib 30 mg, 60 mg or 90 mg up to the recommended dose for up to 12 weeks; instudies for up to 3½ years; in short term acute pain studies for up to 7 days; or in post-marketing experience (see Table 1):
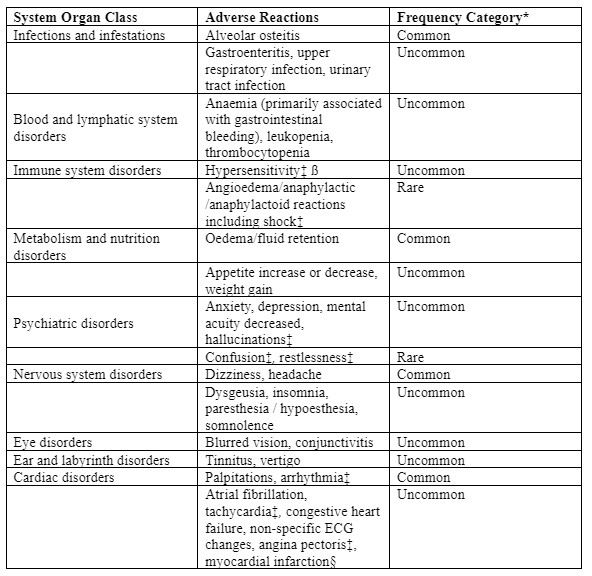
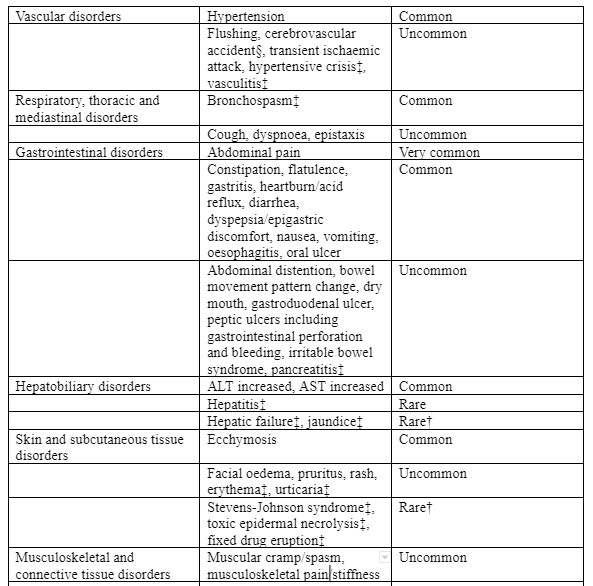
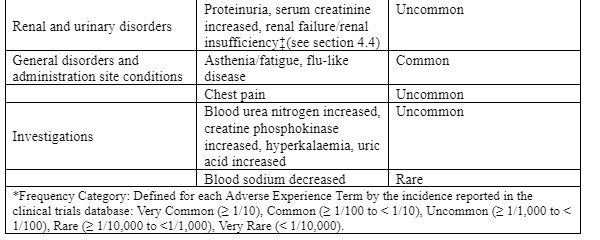

Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
In clinical studies, administration of single doses of etoricoxib up to 500 mg and multiple doses up to 150 mg/day for 21 days did not result in significant toxicity. There have been reports of acute overdosage with etoricoxib, although adverse experiences were not reported in the majority of cases. The most frequently observed adverse experiences were consistent with the safety profile for etoricoxib (e.g. gastrointestinal events, cardiorenal events). In the event of overdose, it is reasonable to employ the usual supportive measures, e.g., remove unabsorbed material from the GI tract, employ clinical monitoring, and institute supportive therapy, if required.
Etoricoxib is not dialysable by haemodialysis; it is not known whether etoricoxib is dialysable by peritoneal dialysis.
5.0 Pharmacological properties
Pharmacodynamic properties
Pharmacotherapeutic group: Anti-inflammatory and anti-rheumatic products, non-steroids, coxibs, ATC code: M01 AH05
Mechanism of Action
Etoricoxib is an oral, selective cyclo-oxygenase-2 (COX-2) inhibitor within the clinical dose range.
Across clinical pharmacology studies, ETOSPEED produced dose-dependent inhibition of COX-2 without inhibition of COX-1 at doses up to 150 mg daily. Etoricoxib did not inhibit gastric prostaglandin synthesis and had no effect on platelet Function.
Cyclooxygenase is responsible for generation of prostaglandins. Two isoforms, COX-1 and COX-2, have been identified. COX-2 is the isoform of the enzyme that has been shown to be induced by pro-inflammatory stimuli and has been postulated to be primarily responsible for the synthesis of prostanoid mediators of pain, inflammation, and fever. COX-2 is also involved in ovulation, implantation and closure of the ductus arteriosus, regulation of renal function, and central nervous system functions (fever induction, pain perception and cognitive function). It may also play a role in ulcer healing. COX-2 has been identified in tissue around gastric ulcers in man but its relevance to ulcer healing has not been established.
Clinical efficacy and safety
Efficacy
In patients with osteoarthritis (OA), etoricoxib 60 mg once daily provided significant improvements in pain and patient assessments of disease status. These beneficial effects were observed as early as the second day of therapy and maintained for up to 52 weeks. Studies with etoricoxib 30 mg once daily demonstrated efficacy superior to placebo over a 12-week treatment period (using similar assessments as the above studies). In a dose ranging study, etoricoxib 60 mg demonstrated significantly greater improvement than 30 mg for all 3 primary endpoints over 6 weeks of treatment. The 30 mg dose has not been studied in osteoarthritis of hands.
In patients with rheumatoid arthritis (RA), etoricoxib 60 mg and 90 mg once daily both provided significant improvements in pain, inflammation, and mobility. In studies evaluating the 60 mg and 90 mg dose, these beneficial effects were maintained over the 12-week treatment periods. In a study evaluating the 60 mg dose compared to the 90 mg dose, etoricoxib 60 mg once daily and 90 mg once daily were both more effective than placebo. The 90 mg dose was superior to the 60 mg dose for Patient Global Assessment of Pain (0-100 mm visual analogue scale), with an average improvement of -2.71 mm (95 % CI: -4.98 mm, -0.45 mm).
In patients experiencing attacks of acute gouty arthritis, etoricoxib 120 mg once daily over an eight-day treatment period, relieved moderate to extreme joint pain and inflammation comparable to indomethacin 50 mg three times daily. Pain relief was observed as early as four hours after initiation of treatment.
In patients with ankylosing spondylitis, etoricoxib 90 mg once daily provided significant improvements in spine pain, inflammation, stiffness and function. The clinical benefit of etoricoxib was observed as early as the second day of therapy after initiation of treatment and was maintained throughout the 52-week treatment period. In a second study evaluating the 60 mg dose compared to the 90 mg dose, etoricoxib 60 mg daily and 90 mg daily demonstrated similar efficacy compared to naproxen 1,000 mg daily. Among inadequate responders to 60 mg daily for 6 weeks, dose escalation to 90 mg daily improved spinal pain intensity score (0-100 mm visual analogue scale) compared to continuing on 60 mg daily, with an average improvement of -2.70 mm (95 % CI: -4.88 mm, -0.52 mm).
In a clinical study evaluating postoperative dental pain, etoricoxib 90 mg was administered once daily for up to three days. In the subgroup of patients with moderate pain at baseline, etoricoxib 90 mg demonstrated a similar analgesic effect to that of ibuprofen 600 mg (16.11 vs. 16.39; P = 0.722), and greater than that of paracetamol/codeine 600 mg/60 mg (11.00; P < 0.001) and placebo (6.84; P < 0.001) as measured by total pain relief over the first 6 hours (TOPAR6). The proportion of patients reporting rescue medication usage within the first 24 hours of dosing was 40.8 % for etoricoxib 90 mg, 25.5 % for ibuprofen 600 mg Q6h, and 46.7 % for paracetamol/codeine 600 mg/60 mg Q6h compared to 76.2 % for placebo. In this study, the median onset of action (perceptible pain relief) of 90 mg etoricoxib was 28 minutes’ after dosing.
Safety
Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) Programme. The MEDAL Programme was a prospectively designed Cardiovascular (CV) Safety Outcomes Programme of pooled data from three randomized, double-blind active comparator-controlled trials, the MEDAL study, EDGE II and EDGE.
The MEDAL Study, was an endpoint driven CV Outcomes study in 17,804 OA and 5,700 RA patients treated with etoricoxib 60 (OA) or 90 mg (OA and RA) or diclofenac 150 mg daily for a mean period of 20.3 months (maximum of 42.3 months, median 21.3 months). In this trial, only serious adverse events and discontinuations due to any adverse events were recorded.
The EDGE and EDGE II studies compared the gastrointestinal tolerability of etoricoxib versus diclofenac. The EDGE study included 7,111 OA patients treated with a dose of etoricoxib 90 mg daily (1.5 times the dose recommended for OA) or diclofenac 150 mg daily for a mean period of 9.1 months (maximum 16.6 months, median 11.4 months). The EDGE study included 4,086 RA patients treated with etoricoxib 90 mg daily or diclofenac 150 mg daily for a mean period of 19.2 months (maximum 33.1 months, median 24 months).
In the pooled MEDAL Programme, 34,701 patients with OA or RA were treated for a mean duration of 17.9 months (maximum 42.3 months, median 16.3 months) with approximately 12,800 patients receiving treatment for more than 24 months. Patients enrolled in the Programme had a wide range of cardiovascular and gastrointestinal risk factors at baseline. Patients with a recent history of myocardial infarction, coronary artery bypass grafting or percutaneous coronary intervention within 6 months preceding enrolment were excluded. Use of gastro-protective agents and low dose aspirin were permitted in the studies.
Overall Safety:
There was no significant difference between etoricoxib and diclofenac in the rate of cardiovascular thrombotic events. Cardiorenal adverse events were observed more frequently with etoricoxib than with diclofenac, and this effect was dose-dependent (see specific results below). Gastrointestinal and hepatic adverse events were observed significantly more frequently with diclofenac than etoricoxib. The incidence of adverse experiences in EDGE and EDGE II and of adverse experiences considered serious or resulting in discontinuation in the MEDAL study was higher with etoricoxib than diclofenac.
Pharmacokinetic properties
Absorption
Orally administered etoricoxib is well absorbed. The absolute bioavailability is approximately 100 %. Following 120 mg once-daily dosing to steady state, the peak plasma concentration (geometric mean Cmax = 3.6 µg/mL) was observed at approximately 1 hour (Tmax) after administration to fasted adults. The geometric mean area under the curve (AUC0-24hr) was 37.8 µg•hr/mL. The pharmacokinetics of etoricoxib are linear across the clinical dose range. Dosing with food (a high-fat meal) had no effect on the extent of absorption of etoricoxib after administration of a 120-mg dose. The rate of absorption was affected, resulting in a 36 % decrease in Cmax and an increase in Tmax by 2 hours. These data are not considered clinically significant. In clinical trials, etoricoxib was administered without regard to food intake.
Distribution
Etoricoxib is approximately 92 % bound to human plasma protein over the range of concentrations of 0.05 to 5 µg/mL. The volume of distribution at steady state (Vdss) was approximately 120 L in humans. Etoricoxib crosses the placenta in rats and rabbits, and the blood-brain barrier in rats.
Biotransformation
Etoricoxib is extensively metabolised with < 1 % of a dose recovered in urine as the parent drug. The major route of metabolism to form the 6'-hydroxymethyl derivative is catalysed by CYP enzymes. CYP3A4 appears to contribute to the metabolism of etoricoxib in vivo. In vitro studies indicate that CYP2D6, CYP2C9, CYP1A2 and CYP2C19 also can catalyse the main metabolic pathway, but their quantitative roles in vivo have not been studied. Five metabolites have been identified in man. The principal metabolite is the 6'-carboxylic acid derivative of etoricoxib formed by further oxidation of the 6'-hydroxymethyl derivative. These principal metabolites either demonstrate no measurable activity or are only weakly active as COX-2 inhibitors. None of these metabolites inhibit COX-1.
Elimination
Following administration of a single 25-mg radio-labelled intravenous dose of etoricoxib to healthy subjects, 70 % of radioactivity was recovered in urine and 20 % in faeces, mostly as metabolites. Less than 2 % was recovered as unchanged drug. Elimination of etoricoxib occurs almost exclusively through metabolism followed by renal excretion. Steady state concentrations of etoricoxib are reached within seven days of once daily administration of 120 mg, with an accumulation ratio of approximately 2, corresponding to a half-life of approximately 22 hours. The plasma clearance after a 25-mg intravenous dose is estimated to be approximately 50 mL/min.
Characteristics in patients
Elderly patients: Pharmacokinetics in the elderly (65 years of age and older) are similar to those in the young.
Gender: The pharmacokinetics of etoricoxib are similar between men and women.
Hepatic impairment: Patients with mild hepatic dysfunction (Child-Pugh score 5-6) administered etoricoxib 60 mg once daily had an approximately 16 % higher mean AUC as compared to healthy subjects given the same regimen. Patients with moderate hepatic dysfunction (Child-Pugh score 7-9) administered etoricoxib 60 mg every other day had similar mean AUC to the healthy subjects given etoricoxib 60 mg once daily; etoricoxib 30 mg once daily has not been studied in this population. There are no clinical or pharmacokinetic data in patients with severe hepatic dysfunction (Child-Pugh score ≥ 10).
Renal impairment: The pharmacokinetics of a single dose of etoricoxib 120 mg in patients with moderate to severe renal insufficiency and patients with end-stage renal disease on haemodialysis were not significantly different from those in healthy subjects. Haemodialysis contributed negligibly to elimination (dialysis clearance approximately 50 mL/min).
Paediatric patients: The pharmacokinetics of etoricoxib in paediatric patients (< 12 years old) have not been studied. In a pharmacokinetic study (n = 16) conducted in adolescents (aged 12 to 17) the pharmacokinetics in adolescents weighing 40 to 60 kg given etoricoxib 60 mg once daily and adolescents > 60 kg given etoricoxib 90 mg once daily were similar to the pharmacokinetics in adults given etoricoxib 90 mg once daily. Safety and effectiveness of etoricoxib in paediatric patients have not been established.
Preclinical safety data
In preclinical studies, etoricoxib has been demonstrated not to be genotoxic. Etoricoxib was not carcinogenic in mice. Rats developed hepatocellular and thyroid follicular cell adenomas at > 2-times the daily human dose [90 mg] based on systemic exposure when dosed daily for approximately two years. Hepatocellular and thyroid follicular cell adenomas observed in rats are considered to be a consequence of rat-specific mechanism related to hepatic CYP enzyme induction. Etoricoxib has not been shown to cause hepatic CYP3A enzyme induction in humans.
In the rat, gastrointestinal toxicity of etoricoxib increased with dose and exposure time. In the 14-week toxicity study etoricoxib caused gastrointestinal ulcers at exposures greater than those seen in man at the therapeutic dose. In the 53- and 106-week toxicity study, gastrointestinal ulcers were also seen at exposures comparable to those seen in man at the therapeutic dose. In dogs, renal and gastrointestinal abnormalities were seen at high exposures.
Etoricoxib was not teratogenic in reproductive toxicity studies conducted in rats at 15 mg/kg/day (this represents approximately 1.5 times the daily human dose [90 mg] based on systemic exposure). In rabbits, a treatment related increase in cardiovascular malformations was observed at exposure levels below the clinical exposure at the daily human dose (90 mg). However, no treatment-related external or skeletal foetal malformations were observed. In rats and rabbits, there was a dose dependent increase in post implantation loss at exposures greater than or equal to 1.5 times the human exposure.
Etoricoxib is excreted in the milk of lactating rats at concentrations approximately two-fold those in plasma. There was a decrease in pup body weight following exposure of pups to milk from dams administered etoricoxib during lactation.
6.0 Pharmaceutical particulars
Incompatibilities
Not applicable
Shelf life
Refer on the pack.
Packaging information
10 Blister strips of 10 tablets in each strip.
Storage and handling instructions
Store below 30°C. Protect from light. Do not freeze.
Keep out of reach of children.
7.0 Patient counselling information
• Ensure the prescribed dose of ETOSPEED 90 is taken as directed.
• Not to exceed the stated recommended daily dose.
• If pregnant or breast-feeding, ask a health professional before use.
• Ask the patient to report any adverse events.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What ETOSPEED is and what it is used for
- What you need to know before you take ETOSPEED
- How to take ETOSPEED
- Possible side effects
- How to store ETOSPEED
- Contents of the pack and other information
1. What ETOSPEED is and what it is used for
What is ETOSPEED?
ETOSPEED contains the active substance etoricoxib. ETOSPEED is one of a group of medicines called selective COX-2 inhibitors. These belong to a family of medicines called non-steroidal anti-inflammatory drugs (NSAIDs).
What is ETOSPEED used for?
- ETOSPEED helps to reduce the pain and swelling (inflammation) in the joints and muscles of people 16 years of age and older with osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and gout.
- ETOSPEED is also used for the short-term treatment of moderate pain after dental surgery in people 16 years of age and older.
What is osteoarthritis?
Osteoarthritis is a disease of the joints. It results from the gradual breakdown of cartilage that cushions the ends of the bones. This causes swelling (inflammation), pain, tenderness, stiffness and disability.
What is rheumatoid arthritis?
Rheumatoid arthritis is a long-term inflammatory disease of the joints. It causes pain, stiffness, swelling, and increasing loss of movement in the joints it affects. It may also cause inflammation in other areas of the body.
What is gout?
Gout is a disease of sudden, recurring attacks of very painful inflammation and redness in the joints. It is caused by deposits of mineral crystals in the joint.
What is ankylosing spondylitis?
Ankylosing spondylitis is an inflammatory disease of the spine and large joints.
2. What you need to know before you take ETOSPEED
Do not take ETOSPEED
- if you are allergic (hypersensitive) to etoricoxib or any of the other ingredients of this medicine (listed in section 6)
- if you are allergic to non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin and COX-2 inhibitors (see Possible Side Effects, section 4)
- if you have a current stomach ulcer or bleeding in your stomach or intestines
- if you have serious liver disease
- if you have serious kidney disease
- if you are or could be pregnant or are breast-feeding (see ‘Pregnancy, breast-feeding, and fertility’)
- if you are under 16 years of age
- if you have inflammatory bowel disease, such as Crohn’s Disease, Ulcerative Colitis, or Colitis
- if you have high blood pressure that has not been controlled by treatment (check with your doctor or nurse if you are not sure whether your blood pressure is adequately controlled)
- if your doctor has diagnosed heart problems including heart failure (moderate or severe types), angina (chest pain)
- if you have had a heart attack, bypass surgery, peripheral arterial disease (poor circulation in legs or feet due to narrow or blocked arteries)
- if you have had any kind of stroke (including mini-stroke, transient ischaemic attack or TIA). Etoricoxib may slightly increase your risk of heart attack and stroke and this is why it should not be used in those who have already had heart problems or stroke.
If you think any of these are relevant to you, do not take the tablets until you have consulted your doctor.
Warnings and precautions
Talk to your doctor or pharmacist before taking ETOSPEED if:
- You have a history of stomach bleeding or ulcers.
- You are dehydrated, for example by a prolonged bout of vomiting or diarrhoea.
- You have swelling due to fluid retention.
- You have a history of heart failure, or any other form of heart disease.
- You have a history of high blood pressure.
- ETOSPEED can increase blood pressure in some people, especially in high doses, and your doctor will want to check your blood pressure from time to time.
- You have any history of liver or kidney disease.
- You are being treated for an infection.
- ETOSPEED can mask or hide a fever, which is a sign of infection. You have diabetes, high cholesterol, or are a smoker.
- These can increase your risk of heart disease.
- You are a woman trying to become pregnant.
- You are over 65 years of age.
If you are not sure if any of the above apply to you, talk to your doctor before taking ETOSPEED to see if this medicine is suitable for you.
ETOSPEED works equally well in older and younger adult patients. If you are over 65 years of age, your doctor will want to appropriately keep a check on you. No dosage adjustment is necessary for patients over 65 years of age.
Children and adolescents
Do not give this medicine to children and adolescents under 16 years of age.
Other medicines and ETOSPEED
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
In particular, if you are taking any of the following medicines, your doctor may want to monitor you to check that your medicines are working properly, once you start taking ETOSPEED:
medicines that thin your blood (anticoagulants), such as warfarin
rifampicin (an antibiotic)
methotrexate (a drug used for suppressing the immune system, and often used in rheumatoid arthritis)
ciclosporin or tacrolimus (drugs used for suppressing the immune system)
lithium (a medicine used to treat some types of depression)
medicines used to help control high blood pressure and heart failure called ACE inhibitors and angiotensin receptor blockers, examples include enalapril and ramipril, and losartan and valsartan
diuretics (water tablets)
digoxin (a medicine for heart failure and irregular heart rhythm)
minoxidil (a drug used to treat high blood pressure)
salbutamol tablets or oral solution (a medicine for asthma)
birth control pills (the combination may increase your risk of side effects)
hormone replacement therapy (the combination may increase your risk of side effects)
aspirin, the risk of stomach ulcers is greater if you take ETOSPEED with aspirin.
- aspirin for prevention of heart attacks or stroke:
ETOSPEED can be taken with low-dose aspirin. If you are currently taking low-dose aspirin to prevent heart attacks or stroke, you should not stop taking aspirin until you talk to your doctor - aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs):
do not take high dose aspirin or other anti-inflammatory medicines while taking ETOSPEED.
ETOSPEED with food and drink
The onset of the effect of ETOSPEED may be faster when taken without food.
Pregnancy, breast-feeding and fertility
Pregnancy
ETOSPEED tablets must not be taken during pregnancy. If you are pregnant or think you could be pregnant, or if you are planning to become pregnant, do not take the tablets. If you become pregnant, stop taking the tablets and consult your doctor. Consult your doctor if you are unsure or need more advice.
Breast-feeding
It is not known if ETOSPEED is excreted in human milk. If you are breast-feeding, or planning to breast-feed, consult your doctor before taking ETOSPEED. If you are using ETOSPEED, you must not breast-feed.
Fertility
ETOSPEED is not recommended in women attempting to become pregnant.
Driving and using machines
Dizziness and sleepiness have been reported in some patients taking ETOSPEED.
Do not drive if you experience dizziness or sleepiness.
Do not use any tools or machines if you experience dizziness or sleepiness.
3. How to take ETOSPEED
Always take this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Do not take more than the recommended dose for your condition. Your doctor will want to discuss your treatment from time to time. It is important that you use the lowest dose that controls your pain and you should not take ETOSPEED for longer than necessary. This is because the risk of heart attacks and strokes might increase after prolonged treatment, especially with high doses.
There are different strengths available for this medicinal product and depending on your disease your doctor will prescribe the tablet strength that is appropriate for you.
The recommended dose is:
Osteoarthritis
The recommended dose is 30 mg once a day, increase to a maximum of 60 mg once a day if needed.
Rheumatoid arthritis
The recommended dose is 60 mg once a day, increased to a maximum of 90 mg once a day if needed.
Ankylosing spondylitis
The recommended dose is 60 mg once a day, increased to a maximum of 90 mg once a day if needed.
Acute pain conditions
Etoricoxib should be used only for the acute painful period.
Gout
The recommended dose is 120 mg once a day which should only be used for the acute painful period, limited to a maximum of 8 days’ treatment.
Postoperative dental surgery pain
The recommended dose is 90 mg once daily, limited to a maximum of 3 days’ treatment.
People with liver problems
If you have mild liver disease, you should not take more than 60 mg a day.
If you have moderate liver disease, you should not take more than 30 mg a day.
Use in children and adolescents
ETOSPEED tablets should not be taken by children or adolescents under 16 years of age.
Elderly
No dose adjustment is necessary for elderly patients. As with other medicines, caution should be exercised in elderly patients.
Method of administration
ETOSPEED is for oral use. Take the tablets once a day. ETOSPEED can be taken with or without food.
If you take more ETOSPEED than you should
You should never take more tablets than the doctor recommends. If you do take too many ETOSPEED tablets, you should seek medical attention immediately.
If you forget to take ETOSPEED
It is important to take ETOSPEED as your doctor has prescribed. If you miss a dose, just resume your usual schedule the following day. Do not take a double dose to make up for the forgotten tablet.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you develop any of these signs you should stop ETOSPEED and talk to your doctor immediately (see What you need to know before you take ETOSPEED section 2):
- shortness of breath, chest pains, or ankle swelling appear or if they get worse
- yellowing of the skin and eyes (jaundice) – these are signs of liver problems
- severe or continual stomach pain or your stools become black
- an allergic reaction- which can include skin problems such as ulcers or blistering, or swelling of the face, lips, tongue, or throat which may cause difficulty in breathing
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 user in 10) Common (affects 1 to 10 users in 100) Uncommon (affects 1 to 10 users in 1,000) Rare (affects 1 to 10 users in 10,000) Very rare (affects less than 1 user in 10,000). The following side effects can occur during treatment with ETOSPEED:
Very Common:
stomach pain
Common:
- dry socket (inflammation and pain after a tooth extraction)
- swelling of the legs and/or feet due to fluid retention (oedema)
- dizziness, headache
- palpitations (fast or irregular heartbeat), irregular heart rhythm (arrhythmia)
- increased blood pressure
- wheezing or shortness of breath (bronchospasms)
- constipation, wind (excessive gas), gastritis (inflammation of the lining of the stomach),heartburn, diarrhoea, indigestion (dyspepsia)/stomach discomfort, nausea,being sick (vomiting), inflammation of the oesophagus, mouth ulcers
- changes in blood tests related to your liver
- bruising
- weakness and fatigue, flu-like illness
Uncommon:
- gastroenteritis (inflammation of the gastrointestinal tract that involves both the stomach and small intestine/stomach flu), upper respiratory infection, urinary tract infection
- changes in laboratory values (decreased number of red blood cells, decreased number of white blood cells, platelets decreased)
- hypersensitivity (an allergic reaction including hives which may be serious enough to require immediate medical attention)
- appetite increases or decreases, weight gain
- anxiety, depression, decreases in mental sharpness; seeing, feeling or hearing things that are not there (hallucinations)
- taste alteration, inability to sleep, numbness or tingling, sleepiness
- blurred vision, eye irritation and redness
- ringing in the ears, vertigo (sensation of spinning while remaining still)
- abnormal heart rhythm (atrial fibrillation), fast heart rate, heart failure, feeling of tightness, pressure or heaviness in the chest (angina pectoris), heart attack
- flushing, stroke, mini-stroke (transient ischaemic attack), severe increase in blood pressure, inflammation of the blood vessels
- cough, breathlessness, nose bleed
- stomach or bowel bloating, changes in your bowel habits, dry mouth, stomach ulcer, inflammation of the stomach lining that can become serious and may lead to bleeding, irritable bowel syndrome, inflammation of the pancreas
- swelling of the face, skin rash or itchy skin, redness of the skin
- muscle cramp/spasm, muscle pain/stiffness
- high levels of potassium in your blood, changes in blood or urine tests relating to your kidney, serious kidney problems
- chest pain
Rare:
- angioedema (an allergic reaction with swelling of the face, lips, tongue and/or throat which may cause difficulty in breathing or swallowing, which may be serious enough to require immediate medical attention)/anaphylactic/anaphylactoid reactions including shock (a serious allergic reaction that requires immediate medical attention)
- confusion, restlessness
- liver problems (hepatitis)
- low blood levels of sodium
- liver failure, yellowing of the skin and/or eyes (jaundice)
- severe skin reactions
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to Store Etospeed
Store in a cool, dry & dark place.
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton. The expiry date refers to the last day of that month. Blisters: Store in the original package in order to protect from moisture.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What ETOSPEED contains
- The active substance is etoricoxib. Each film coated tablet contains 90 mg of etoricoxib.
- 10 Blister strips of 10 tablets in each strip.