Eucalmin Capsules
Therapy Area
Vitamins/Minerals Supplements
1.0 Generic Name
Calcitriol, Omega-3 Acids, Mecobalamin, Folic Acid, Boron & Calcium Capsules
2.0 Qualitative and quantitative composition
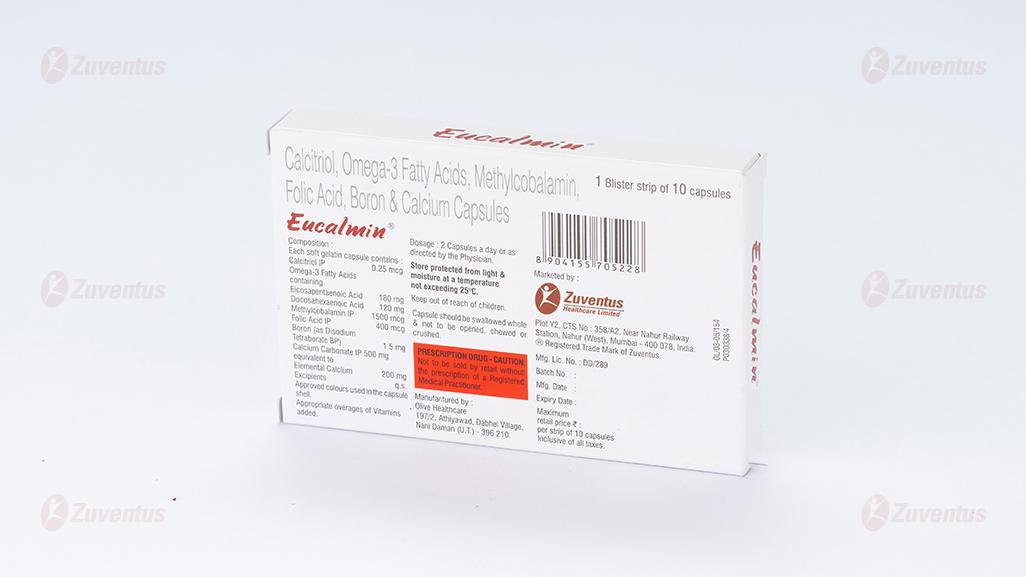
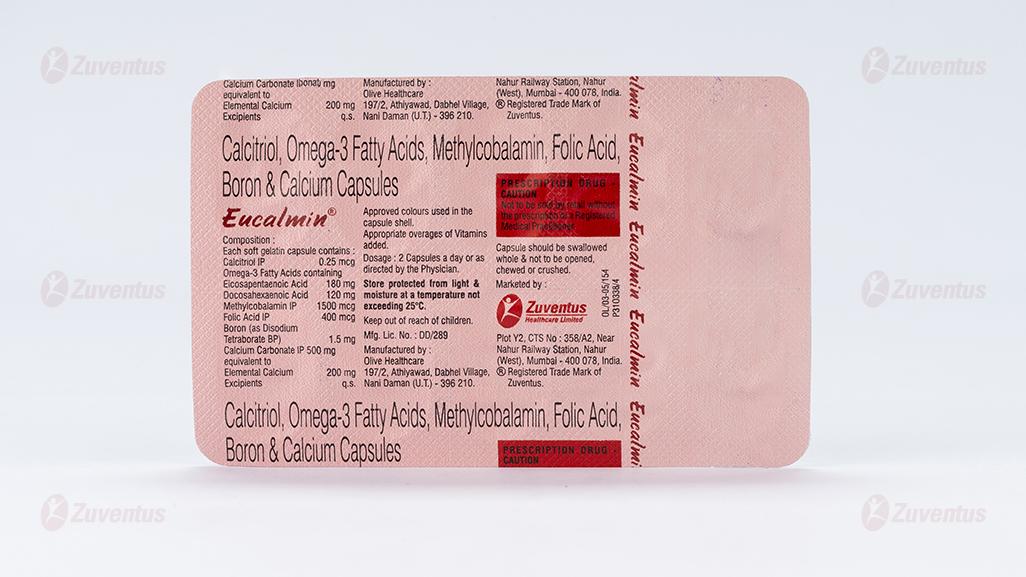
Each soft gelatin capsule contains:
Calcitriol IP 0.25 mcg
Omega-3 Acids BP containing:
Eicosapentaenoic Acid 180 mg
Docosahexaenoic Acid 120 mg
Mecobalamin IP 1500 mcg
Folic Acid IP 400 mcg
Boron 1.5 mg
(As Disodium Tetraborate BP)
Calcium Carbonate IP 500 mg
equivalent to Elemental Calcium 200 mg
Excipients q.s.
Approved colours used in the capsule shells.
3.0 Dosage form and strength
Soft Gelatin Capsule
Calcitriol (0.25 mcg), Omega-3 Acids (EPA 180 mg, DHA120 mg), Mecobalamin (1500 mcg), Folic Acid (400 mcg), Boron (1.5 mg), Calcium (200 mg).
4.0 Clinical Particulars
4.1 Therapeutic indication
For the treatment:
- hypocalcaemia and/or osteoporosis.
- vitamin and calcium deficiency states in adults
- post-menopausal osteoporosis
4.2 Posology and method of administration
Average adult dose is 1 capsule once daily. For treatment of post-menopausal osteoporosis: 1 capsule twice daily. Eucalmin® capsules are for oral administration only.
4.3 Contraindications
Eucalmin® capsules is contraindicated:
- in patients with known hypersensitivity to calcitriol (or drugs of the same class) and any of the component.
- in all diseases associated with hypercalcaemia for example from myeloma, bone metastases or other malignant bone disease, sarcoidosis; primary hyperparathyroidism and vitamin D overdosage.
- Severe renal failure.
- in patients with evidence of metastatic calcification
- if there is evidence of vitamin D toxicity.
- hypervitaminosis from any vitamin contained in this formulation,
- Relative contraindications are osteoporosis due to prolonged immobilisation, renal stones, severe hypercalciuria.
- If allergic to fish oil, as Eucalmin® capsules contains (Omega-3 acids).
4.4 Special warnings and precautions for use
There is a close correlation between treatment with calcitriol and the development of hypercalcaemia.
All other vitamin D compounds and their derivatives, including proprietary compounds or foodstuffs which may be “fortified” with vitamin D, should be withheld during treatment with Eucalmin® capsules.
An abrupt increase in calcium intake as a result of changes in diet (e.g. increased consumption of dairy products) or uncontrolled intake of calcium preparations may trigger hypercalcaemia. Patients and their families should be advised that strict adherence to the prescribed diet is mandatory and they should be instructed on how to recognise the symptoms of hypercalcaemia.
As soon as the serum calcium levels rise to 1 mg/100 ml (250 µ mol/l) above normal (9-11 mg/100 ml or 2250-2750 µ mol/l), or serum creatinine rises to >120 µ mol/l, treatment with Eucalmin® capsules should be stopped immediately until normocalcaemia ensues.
Immobilised patients, e.g. those who have undergone surgery, are particularly exposed to the risk of hypercalcaemia.
Calcitriol increases inorganic phosphate levels in serum. While this is desirable in patients with hypophosphataemia, caution is called for in patients with renal failure because of the danger of ectopic calcification. In such cases, the plasma phosphate level should be maintained at the normal level (2-5 mg/100 ml or 0.65-1.62 mmol/l) by the oral administration of appropriate phosphate-binding agents and low phosphate diet.
The serum calcium times phosphate (Ca x P) product should not be allowed to exceed 70 mg2/dl2.
Patients with vitamin D-resistant rickets (familial hypophosphataemia) who are being treated with Eucalmin® capsules must continue their oral phosphate therapy. However, possible stimulation of intestinal absorption of phosphate by Eucalmin® capsules should be taken into account since this effect may modify the need for phosphate supplementation.
Since calcitriol is the most effective vitamin D metabolite available, no other vitamin D preparation should be prescribed during treatment with Eucalmin® capsules, thereby ensuring that the development of hypervitaminosis D is avoided.
If the patient is switched from a long acting vitamin D preparation (e.g. ergocalciferol (vitamin D2) or colecalciferol) to calcitriol, it may take several months for the ergocalciferol level in the blood to return to the baseline value, thereby increasing the risk of hypercalcaemia.
Patients with normal renal function who are taking Eucalmin® capsules should avoid dehydration. Adequate fluid intake should be maintained.
In patients with normal renal function, chronic hypercalcaemia may be associated with an increase in serum creatinine.
4.5 Drugs interactions
Calcium and Calcitriol
- Dietary instructions, especially concerning calcium supplements, should be strictly observed, and uncontrolled intake of additional calcium-containing preparations avoided.
- Concomitant treatment with a thiazide diuretic increases the risk of hypercalcaemia. Calcitriol dosage must be determined with care in patients undergoing treatment with digitalis, as hypercalcaemia in such patients may precipitate cardiac arrhythmias (see section 4.4).
- A relationship of functional antagonism exists between vitamin D analogues, which promote calcium absorption, and corticosteroids, which inhibit it.
- Magnesium-containing drugs (e.g. antacids) may cause hypermagnesaemia and should therefore not be taken during therapy with Eucalmin® capsules by patients on chronic renal dialysis.
- Since Eucalmin® capsules also has an effect on phosphate transport in the intestine, kidneys and bones, the dosage of phosphate-binding agents must be adjusted in accordance with the serum phosphate concentration (normal values: 2-5 mg/100 ml, or 0.65-1.62 mmol/l).
- Patients with vitamin D-resistant rickets (familial hypophosphataemia) should continue their oral phosphate therapy. However, possible stimulation of intestinal phosphate absorption by calcitriol should be taken into account since this effect may modify the requirement for phosphate supplements.
- Bile acid sequestrants including cholestyramine and sevelamer can reduce intestinal absorption of fat-soluble vitamins and therefore may impair intestinal absorption of calcitriol.
- Certain antiretroviral agents: Decreased vitamin D levels have been associated with, e.g., efavirenz and zidovudine. Decreased formation of the active vitamin D metabolite has been associated with protease inhibitors.
Folic acid
- Anticonvulsants (phenytoin, fosphenytoin, phenobarbital, primidone): Folic acid supplementation can decrease the anticonvulsant serum concentration and increase seizure risk.
- Aspirin (high dose therapy): Can reduce folic acid levels by increasing urinary excretion
- Certain anticonvulsants (e.g., phenytoin, carbamazepine, phenobarbital, valproate): Can cause folate, pyridoxine and vitamin D deficiencies
- Folate antagonists, e.g., methotrexate, sulfasalazine, pyrimethamine, triamterene, trimethoprim, and high doses of tea catechins: Block the conversion of folate to its active metabolites and reduce the effectiveness of supplementation
- Folate antimetabolites (methotrexate, raltitrexed): Folic acid supplementation can decrease the antimetabolite effects
Mecobalamin
Chloramphenicol: Can inhibit the haematological response to vitamin B12 therapy.
Omega-3 fatty acids
Omega-3 fatty acids can interact with several medications, which may affect their efficacy or increase the risk of side effects:
- Anticoagulants and Antiplatelet Drugs: Omega-3s can enhance the effects of blood-thinning medications like warfarin (Coumadin) and aspirin, increasing the risk of bleeding.
- Blood Pressure Medications: Omega-3 supplements might slightly lower blood pressure, which can enhance the effects of antihypertensive drugs, potentially leading to hypotension.
- Contraceptive Drugs: Some studies suggest that contraceptive drugs may reduce the effectiveness of omega-3 supplements.
- Orlistat: This weight-loss medication can reduce the absorption of omega-3 fatty acids.
- Vitamin E: Taking omega-3 supplements can reduce vitamin E levels in the body.
Boron
Boron is generally considered safe and does not have many known drug interactions. However, there are a few mild interactions to be aware of:
- Estrogens: Boron can interact with various forms of estrogen, including conjugated estrogens, estradiol, and synthetic Estrogens.
- Hormone Replacement Therapy: boron might affect the levels of hormones in body.
4.6 Use in special populations
Pregnancy
The safety of Eucalmin® capsules during pregnancy has not been established.
Supravalvular aortic stenosis has been produced in foetuses by near-fatal oral doses of vitamin D in pregnant rabbits. There is no evidence to suggest that vitamin D is teratogenic in humans even at very high doses. Eucalmin® capsules should be used during pregnancy only if the benefits outweigh the potential risk to the foetus.
Breast-feeding
It should be assumed that exogenous calcitriol passes into breast milk. In view of the potential for hypercalcaemia in the mother and for adverse reactions from Eucalmin® capsules in nursing infants, mothers may breastfeed while taking Eucalmin® capsules, provided that the serum calcium levels of the mother and infant are monitored.
4.7 Effects on ability to drive and use machines
On the basis of the pharmacodynamic profile of reported adverse events, this product is presumed to be safe or unlikely to adversely affect such activities.
4.8 Undesirable effects
The adverse reactions listed below reflect the experience from investigational studies of Eucalmin® capsules, and the post-marketing experience.
The most commonly reported adverse reaction was hypercalcaemia.
The ADRs listed in Table 1 are presented by system organ class and frequency categories, defined using the following convention: Very common (≥ 1/10); common (≥ 1/100 to <1/10); uncommon (≥ 1/1,000 to <1/100); rare (≥ 1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.

Since calcitriol exerts vitamin D activity, adverse effects may occur which are similar to those found when an excessive dose of vitamin D is taken, i.e. hypercalcaemia syndrome or calcium intoxication (depending on the severity and duration of hypercalcaemia).
Occasional acute symptoms include decreased appetite, headache, nausea, vomiting, abdominal pain or abdominal pain upper and constipation.
Because of the short biological half-life of calcitriol, pharmacokinetic investigations have shown normalisation of elevated serum calcium within a few days of treatment withdrawal, i.e. much faster than in treatment with vitamin D3 preparations.
Chronic effects may include muscular weakness, weight decreased, sensory disturbances, pyrexia, thirst, polydipsia, polyuria, dehydration, apathy, growth retardation and urinary tract infections.
In concurrent hypercalcaemia and hyperphosphataemia of > 6 mg/100 ml or > 1.9 mmol/l, calcinosis may occur; this can be seen radiographically.
Hypersensitivity reactions including rash, erythema, pruritus and urticaria may occur in susceptible individuals.
Laboratory Abnormalities
In patients with normal renal function, chronic hypercalcaemia may be associated with a blood creatinine increase.
Post Marketing
The number of adverse effects reported from clinical use of Eucalmin® capsules in all indications is very low with each individual effect, including hypercalcaemia, occurring at a rate of 0.001 % or less.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Treatment of asymptomatic hypercalcaemia.
Since calcitriol is a derivative of vitamin D, the symptoms of overdose are the same as for an overdose of vitamin D. Intake of high doses of calcium and phosphate together with Eucalmin® capsules may give rise to similar symptoms. The serum calcium times phosphate (Ca x P) product should not be allowed to exceed 70 mg2 / dl2. A high calcium level in the dialysate may contribute to the development of hypercalcaemia.
Acute symptoms of vitamin D intoxication: anorexia, headache, vomiting, constipation.
Chronic symptoms: dystrophy (weakness, loss of weight), sensory disturbances, possibly fever with thirst, polyuria, dehydration, apathy, arrested growth and urinary tract infections. Hypercalcaemia ensues, with metastatic calcification of the renal cortex, myocardium, lungs and pancreas.
The following measures should be considered in treatment of accidental overdosage: immediate gastric lavage or induction of vomiting to prevent further absorption. Administration of liquid paraffin to promote faecal excretion. Repeated serum calcium determinations are advisable. If elevated calcium levels persist in the serum, phosphates and corticosteroids may be administered and measures instituted to bring about adequate diuresis.
Hypercalcaemia at higher levels (>3.2 mmol/L) may lead to renal insufficiency particularly if blood phosphate levels are normal or elevated due to impaired renal function.
Should hypercalcaemia occur following prolonged treatment, Eucalmin® capsules should be discontinued until plasma calcium levels have returned to normal. A low-calcium diet will speed this reversal. Eucalmin® capsules can then be restarted at a lower dose or given in the same dose but at less frequent intervals than previously.
In patients treated by intermittent haemodialysis, a low concentration of calcium in the dialysate may also be used. However, a high concentration of calcium in the dialysate may contribute to the development of hypercalcaemia.
5.0 Pharmacological Properties
5.1 Pharmacodynamic properties
Calcitriol
Calcitriol, the active form of vitamin D, works by binding to the vitamin D receptor (VDR) in various tissues such as the intestines, kidneys, and bones. This active form of vitamin D3, crucial for stimulating intestinal calcium transport and regulating bone mineralization.
-Calcitriol enhances the absorption of calcium and phosphate from the gastrointestinal tract. -It increases the reabsorption of calcium in the kidneys, reducing calcium loss through urine. -Calcitriol stimulates the release of calcium from bones into the bloodstream. -By binding to VDR, calcitriol acts as a transcription factor, regulating the expression of various genes involved in calcium and phosphate metabolism. This multi-faceted approach helps maintain adequate calcium levels in the blood, which is crucial for various bodily functions, including bone health and muscle function.
In osteoporosis, calcitriol enhances calcium absorption, increases circulating calcitriol levels, and reduces vertebral fracture frequency. Its effects onset and reversal are quicker than other vitamin D compounds, allowing for more precise dosage adjustments and easier management of potential overdosage.
Calcium Carbonate
Calcium is the most abundant mineral in the body. The major function of calcium is in the formation of the major inorganic bone component, hydroxyapatite. Approximately 60% of the weight of bone is due to the presence of calcium-rich mineral deposits. During menopause calcium absorption is decreased. If this calcium is not replaced by the dietary supply, plasma calcium is maintained by resorption of bone under the action of parathyroid hormone (PTH). If there is a continual low intake of calcium, or if intestinal absorption is impaired over a prolonged period, bone resorption will cause a severe decrease in bone mass and this dietary imbalance, together with a number of other factors, contributes to an increased risk of osteoporosis.
Various interventional studies which used a combined calcium and vitamin D, improves bone mineral density (BMD), reduces fracture risk, improves muscle strength there by reducing frequent falls.
Omega-3 Acids (Eicosapentaenoic Acid, Docosahexaenoic Acid)
Polyunsaturated fatty acids (PUFAs), specifically linoleic acid (n-6) and alpha-linolenic acid (n-3), are essential for human growth and function. Only plants and some fish can synthesize these fatty acids from shorter chain fats. While vegetable oils and dairy fats contain short- and medium-chain fatty acids, fish oils and certain plants are rich in n-3 fatty acids.
Omega-3 Acids (ω-3 PUFAs) are believed to support bone health through several mechanisms, including reducing inflammatory cytokines, modulating prostaglandin E2 (PGE2) production, enhancing calcium transport, and decreasing urinary calcium excretion. Research indicates that EPA and DHA can inhibit bone loss and resorption, increase calcium absorption, and improve bone mineral density (BMD).
Additionally, Omega-3 Acids may serve as competitive inhibitors in eicosanoid biosynthesis, leading to lower production of pro-inflammatory cytokines, particularly in conditions like rheumatoid arthritis. Studies show that n-3 PUFA supplements can have beneficial effects on joint diseases by suppressing pro-inflammatory factors like IL-1, IL-2, and TNF in cartilage tissue.
Mecobalamin and Folic acid
Elevated homocysteine levels and low vitamin B12 and folate status are linked to lower bone mass and higher fracture risk in both independent and frail elderly individuals. Vitamin B12 stimulates osteoblast proliferation and is crucial for their maturation. In contrast, high homocysteine can lead to skeletal abnormalities and decreased bone mineral density (BMD) by disrupting collagen cross-linking, which is essential for bone strength.
Research indicates a stronger relationship between serum folate levels and BMD compared to other osteoporosis risk factors. Low folate often reflects an inadequate diet, leading to deficiencies in nutrients necessary for bone mineralization. Folate is vital for various cellular processes, including DNA protection and reducing oxidative stress.
Combining folate and vitamin B12 supplementation is considered a safe and effective approach to lowering fracture risk in elderly patients.
Boron
Boron, a trace metalloid, is found in high levels in plant-based foods like dried fruits, nuts, and leafy greens. Daily boron needs likely range from 1 to 20 mg, influenced by factors like stress and the intake of other minerals. It plays a role in calcium and magnesium metabolism and is concentrated in bone, spleen, and thyroid, suggesting its importance in bone health and hormone regulation.
Low boron diets are linked to reduced testosterone levels, and supplements may enhance testosterone and estradiol levels, particularly in postmenopausal women. Boron is often marketed to athletes and bodybuilders for its potential benefits in increasing testosterone, muscle mass, and strength. Additionally, boron supplementation can reduce urinary losses of essential minerals like calcium, zinc, and magnesium, which may help combat bone demineralization in conditions like osteoporosis and osteoarthritis.
Boron is particularly significant when dietary vitamin D is insufficient, supporting optimal bone health and metabolic functions.
5.3 Pharmacokinetic properties
Calcitriol
Absorption: Calcitriol is rapidly absorbed from the intestine. Peak serum concentrations following a single oral dose of 0.25-1µ g Calcitriol in healthy subjects were found within 2-6 hours. After a single oral dose of 0.5 mcg Calcitriol in healthy subjects, the average serum concentrations of calcitriol rose from a baseline value of 40.0 ± 4.4 pg/ml to 60.0 ± 4.4 pg/ml after two hours, and then fell to 53.0 ± 6.9 after four hours, to 50.0 ± 7.0 after eight hours, to 44 ± 4.6 after twelve hours and to 41.5 ± 5.1 pg/ml after 24 hours.
Distribution: During transport in the blood at physiological concentrations, calcitriol is mostly bound to a specific vitamin D binding protein (DBP), but also, to a lesser degree, to lipoproteins and albumin. At higher blood calcitriol concentrations, DBP appears to become saturated, and increased binding to lipoproteins and albumin occurs.
Biotransformation: Calcitriol is hydroxylated and oxidised in the kidney and in the liver by a specific cytochrome P450 enzyme: CYP24A1. Several metabolites with different degrees of vitamin D activity have been identified.
Elimination: The elimination half-life of calcitriol in plasma ranges between 5 to 8 hours. However, the pharmacological effect of a single dose of calcitriol lasts at least 4 days. The elimination and absorption kinetics of calcitriol remain linear in a very broad dose range and up to 165 µ g single oral dose. Calcitriol is excreted in the bile and may undergo an enterohepatic circulation.
Mecobalamin
Vitamin B12 is extensively bound to specific plasma proteins called transcobalamins; transcobalamin II appears to be involved in the rapid transport of the cobalamins to tissues.
Vitamin B12 diffuses across the placenta and also appears in breast milk. Vitamin B12 is stored in the liver, excreted in the bile, and undergoes extensive enterohepatic recycling. Part of a dose is excreted in the urine, most of it in the first 8 hours; urinary excretion, however, accounts for only a small fraction in the reduction of total body stores acquired by dietary means.
Folic Acid
Folic acid is converted to the metabolically active form 5-methyltetrahydrofolate in the plasma and liver. Folate is distributed into breast milk. The principal storage site of folate is the liver; it is also actively concentrated in the CSF. Folate undergoes enterohepatic circulation. Folate metabolites are eliminated in the urine and folate in excess of body requirements is excreted unchanged in the urine.
Boron
Absorption: Boron compounds, such as boric acid, are readily and completely absorbed when administered orally. Distribution: Once absorbed, boron is distributed throughout the body water via passive diffusion. It tends to accumulate in tissues like the liver, kidneys, and bones. Metabolism: Boron is not significantly metabolized in the body. It remains largely in its original form. Excretion: Boron is primarily excreted through the urine. The elimination half-life can vary, but it is generally rapid, with boron being cleared from the blood within hours
Omega-3 fatty acids
The pharmacokinetics of omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), involve several key processes: Absorption: Omega-3 fatty acids are absorbed in the small intestine. They are typically ingested as triglycerides or ethyl esters, which are then hydrolyzed by pancreatic lipase to free fatty acids and monoglycerides.
Distribution: After absorption, omega-3 fatty acids are incorporated into chylomicrons and transported via the lymphatic system into the bloodstream. They are then distributed to various tissues, including the liver, heart, brain, and adipose tissue. Metabolism: In the liver, omega-3 fatty acids undergo beta-oxidation to produce energy or are re-esterified into triglycerides and phospholipids. They can also be converted into bioactive metabolites like eicosanoids, resolvins, and protectins, which have anti-inflammatory and cardioprotective effects.
Excretion: Omega-3 fatty acids and their metabolites are primarily excreted via the urine. Some are also excreted in the feces. Bioavailability: The bioavailability of omega-3 fatty acids can be influenced by factors such as the form of the supplement (triglyceride or ethyl ester), the presence of other dietary fats, and individual differences in digestion and absorption.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Subchronic toxicity studies in rats and dogs indicated that calcitriol at an oral dose of 20 ng/kg/day (twice the usual human dosage) for up to 6 months produced no or minimal adverse effects. A dose of 80 ng/kg/day (8 times the usual human dosage) for up to 6 months produced moderate adverse effects; changes seen appeared to be primarily the result of prolonged hypercalcaemia.
Reproductive toxicity studies in rats indicated that oral doses up to 300 ng/kg/day (30 times the usual human dose) did not adversely affect reproduction. In rabbits, multiple foetal abnormalities were observed in two litters at an oral maternally toxic dose of 300 ng/kg/day and one litter at 80 ng/kg/day, but not at 20 ng/kg/day (twice the usual human dose). Although there were no statistically significant differences between treated groups and controls in the numbers of litters or foetuses showing abnormalities, the possibility that these findings were due to calcitriol administration could not be discounted.
7.0 Description
Calcitriol is a synthetic vitamin D analog which is active in the regulation of the absorption of calcium from the gastrointestinal tract and its utilization in the body. It has a calculated molecular weight of 416.65.
Chemically, calcitriol is 9, 10-seco(5Z,7E)-5,7,10(19) cholestatriene-1α, 3β, 25-triol and has the following structural formula:

Calcium Carbonate

Methylcobalamin appears as dark red crystals or crystalline powder. It is sparingly soluble in water, slightly soluble in ethanol and practically insoluble in acetonitrile.
Molecular Weight: 1344.38 g/mol.
Molecular Formula: C63H91CoN13O14P.
Chemical Name: Methyl-5, 6-dimethylbenzimidazolylcobalamin

Folic acid
Folic acid is a yellow, yellow-brownish, or yellowish orange, odourless crystalline powder.
Very slightly soluble in water; insoluble in alcohol, in acetone, in chloroform, and in ether. Molecular Weight: 441.40 g/mol. Molecular Formula: C19H19N7O6. Chemical Name: (2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino} phenyl) formamido] pentanedioic acid.
Structural Formula:

Boron
Disodium tetraborate, commonly known as borax, is a boron compound with the chemical formula Na₂B₄O₇·10H₂O. It is a white, powdery substance that dissolves in water to form a basic solution.

Omega-3 fatty acids
Omega-3 fatty acids are polyunsaturated fatty acids (PUFAs) with a double bond at the third carbon atom from the end of the carbon chain.
Omega-3 Fatty Acid is any fatty acid that contains an unsaturated bond originating from the 3rd carbon from the methyl end. Omega-3 fatty acids do not occur naturally with chain lengths shorter than 16 carbon units.
The three types of omega-3 fatty acids involved in human physiology are α-linolenic acid (ALA) (found in plant oils), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (both commonly found in fish oil that originally come from microalgae that is further consumed by phytoplankton, a source of diet for fish).
8.0 Pharmaceutical Properties
8.1 Incompatibilities
None
8.2 Shelf-life
Refer on pack
8.3 Packaging information
1 Blister strip of 10 capsules
8.4 Storage and handing instructions
Store protected from light and moisture at a temperature not exceeding 25°C. Keep out of reach of children.
9.0 Patient Counselling Information
- Capsule should be swallowed whole and not to be opened, chewed or crushed.
- The patient and his or her parents or spouse should be informed about compliance with dosage instructions, adherence to instructions about diet and calcium supplementation and avoidance of the use of unapproved non-prescription drugs. Patients should also be carefully informed about the symptoms of hypercalcemia.
- The effectiveness of Eucalmin® capsules therapy is predicated on the assumption that each patient is receiving an adequate daily intake of calcium. Patients are advised to have a dietary intake of calcium at a minimum of 600 mg daily. The U.S. RDA for calcium in adults is 800 mg to 1200 mg.
12.0 Date of revision of the text
26th September 2024
Read this entire leaflet carefully before you start taking this medicine because it contains important information for you
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
1. What Eucalmin® is and what it is used for
Eucalmin® is a combination of vitamins and mineral supplements prescribed to treat vitamin and other nutritional deficiencies. Eucalmin® promotes bone and joint health, and improves mobility, maintains normal calcium level in body.
Calcitriol, the active form of vitamin D, enhances intestinal calcium and phosphate absorption, increases renal calcium reabsorption, and stimulates bone calcium release, crucial for maintaining blood calcium levels and supporting bone health. Calcium carbonate is essential for bone structure, and its deficiency can lead to osteoporosis, particularly after menopause. Omega-3 fatty acids (EPA and DHA) support bone health by reducing inflammation and enhancing calcium absorption. Mecobalamin and folic acid help lower homocysteine levels, crucial for osteoblast function and bone density. Boron, found in plant-based foods, aids in calcium and magnesium metabolism and may improve testosterone levels. Boron has an important function in the incorporation of calcium into joint cartilage, which helps to avoid joint pain and degeneration.
Together, these nutrients play significant roles in bone mineral density and fracture risk reduction, especially in the elderly.
Uses: Eucalmin® capsules are used for:
- Treating hypocalcaemia (low calcium levels) and osteoporosis.
- Addressing vitamin and calcium deficiencies in adults.
- Managing post-menopausal osteoporosis.
2. What you need to know before you take Eucalmin®
Do not take Eucalmin® if you:
Are allergic to calcitriol or any other ingredients in this medicine.
Have hypercalcaemia or conditions associated with high calcium levels.
Have severe renal failure or evidence of metastatic calcification.
Have vitamin D toxicity or hypervitaminosis from any vitamin in this formulation.
Are allergic to fish oil (contains Omega-3 acids).
Warnings and precautions:
Avoid other vitamin D compounds during treatment.
Monitor calcium intake to prevent hypercalcaemia.
Patients with renal failure should maintain normal plasma phosphate levels.
Avoid dehydration and maintain adequate fluid intake.
Use During Pregnancy and Lactation:
Pregnancy: Teratogenic Effects: Pregnancy Category C
The safety of Eucalmin® capsules during pregnancy has NOT been established.
Breastfeeding: Calcitriol may pass into breast milk. Mothers may breastfeed while taking Eucalmin® capsules, provided that the serum calcium levels of both mother and infant are monitored.
Effects on Ability to Drive and Use Machines:
Based on the pharmacodynamic profile, Eucalmin® capsules are presumed to be safe and unlikely to adversely affect your ability to drive or use machines.
Drug –drug Interactions:
- Avoid taking additional calcium supplements without consulting your doctor.
- Inform your doctor if you are taking diuretics, digitalis, antacids, anticonvulsants, or any other medications.
3. How to take Eucalmin®
Adults: 1 capsule once daily.
Post-menopausal osteoporosis: 1 capsule twice daily.
Swallow the capsule whole, do not chew or crush.
4. Possible side effects
Common side effects: Hypercalcaemia, decreased appetite, nausea, vomiting, constipation
Uncommon side effects: Headache, muscular weakness, sensory disturbances
Rare side effects: Cardiac arrhythmias, psychiatric disturbances
Not known: Hypersensitivity reactions, rash, erythema, pruritus
Reporting of side effects: If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.You can also report side effects directly: Website: www.zuventus.com in and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this product.
5. How to store Eucalmin®
Store protected from light and moisture at a temperature not exceeding 25°C.
Keep out of reach of children.
6. Contents of the pack and other information
What Eucalmin® contains:
Calcitriol 0.25 mcg
Omega-3 Acids (EPA 180 mg, DHA 120 mg)
Mecobalamin 1500 mcg
Folic Acid 400 mcg
Boron 1.5 mg (as Disodium Tetraborate)
Calcium Carbonate 500 mg (equivalent to Elemental Calcium 200 mg)
Pack size:
1 blister strips of 10 tablets each