EvaNew Tablets
Therapy Area
Women's Health
1.0 Generic name
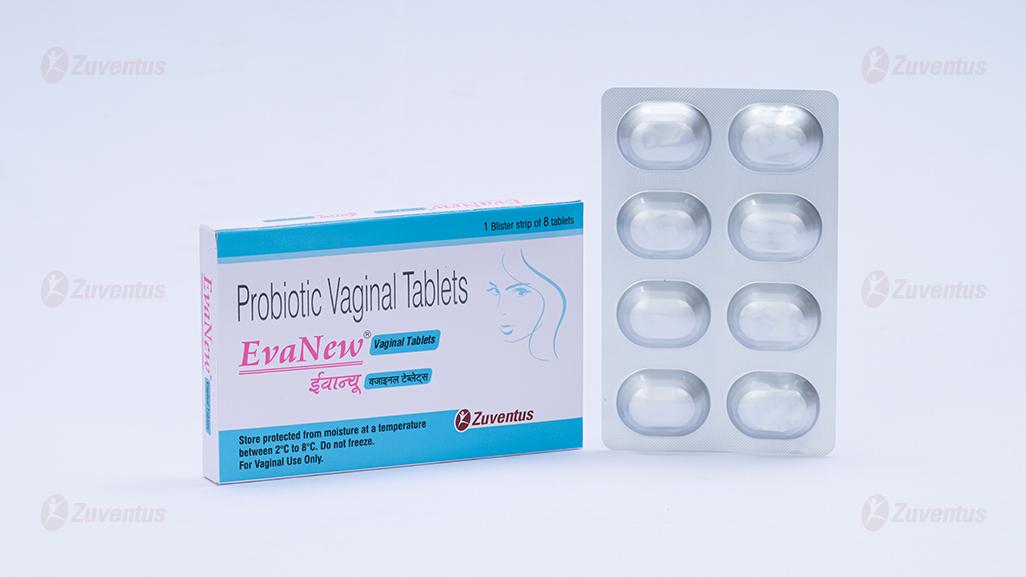
Probiotic Vaginal Tablets
2.0 Qualitative and quantitative composition
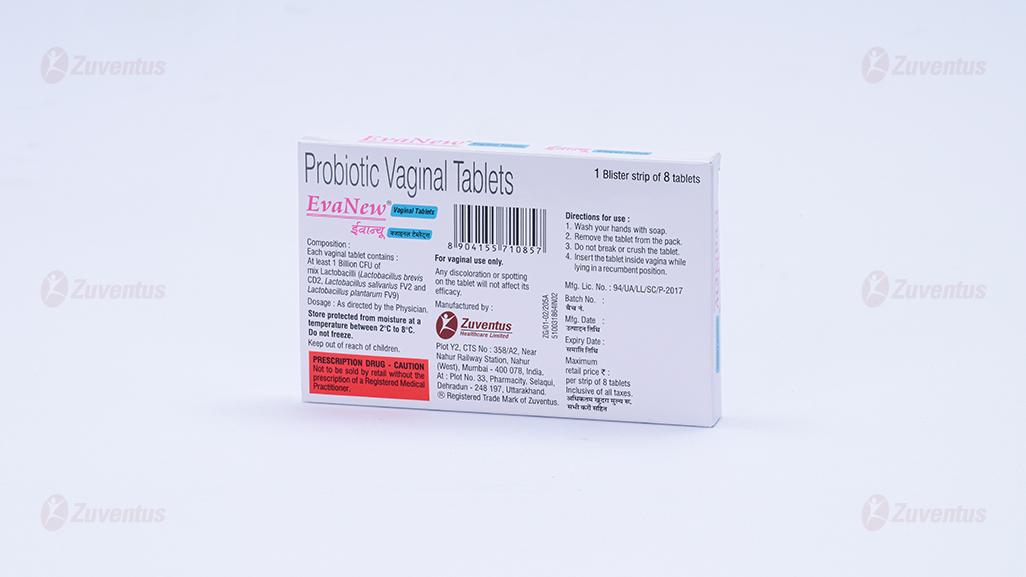
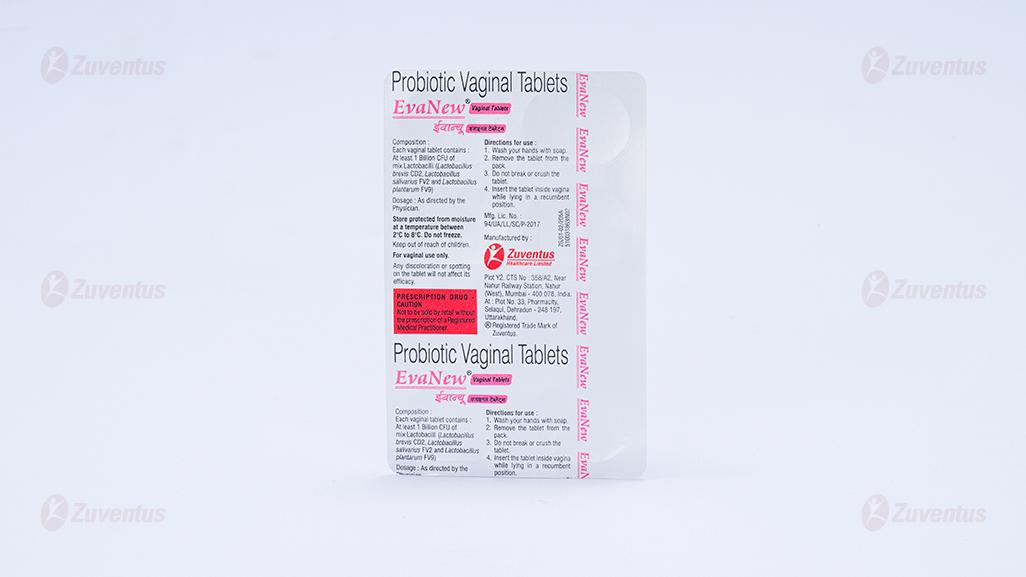
Each vaginal tablet contains:
At least 1 Billion CFU of Mix Lactobacilli: L. brevis CD2, L. salivarius FV2 & L. plantarum FV9.
3.0 Dosage form and strength
Vaginal tablet
1 Billion CFU
4.0 Clinical particulars
4.1 Therapeutic Indication
- Helps to maintain normal vaginal flora and restore healthy vaginal pH, in general, and after clinical treatment of opportunistic infections (Vulvovaginal candidiasis, protozoal and viral infections).
- Treatment of Bacterial Vaginosis and prevention of relapse of Bacterial Vaginosis.
4.2 Posology and method of administration
1 tablet intra-vaginal at bedtime for 8 days every month preferably from 8 day of menstrual cycle for 3 consecutive months.
Method of Administration:
- Wash your hands with soap.
- Remove the tablet from the pack.
- Do not break or crush the tablet
- insert the tablet inside the vagina while lying in a recumbent position.
4.3 Contraindications
Known hypersensitivity to any of the ingredients mentioned under COMPOSITION section.
4.4 Special warnings and precautions for use
- Do not use if the packaging is broken or damaged
- Avoid douching during treatment
- Do not ingest (Only for Vaginal Use).
- Avoid use of intra-vaginal antibiotics during treatment.
- Compatible with condoms and other physical barrier methods without any interference in intercourse.
4.5 Drugs interactions
EvaNew vaginal tablet can be taken with any other pharmaceutical products without interference.
4.6 Use in special populations
Pregnancy and Lactation:
No adverse events have been reported with usage of EvaNew vaginal tablet in pregnant and lactating women.
4.7 Effects on ability to drive and use machines
None Anticipated
4.8 Undesirable effects
- Non-teratogenic.
- No adverse effects reported on reproductive health and fertility in animals.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
No over dosage has been reported for EvaNew vaginal tablet.
5.0 Pharmacological properties
5.1 Pharmacodynamic Properties
EvaNew vaginal tablet is a novel product, possessing anti-microbial and anti-inflammatory activities. An optimal concentration of the three strains in EvaNew vaginal tablet is designed based on their adhesive properties, H2O2 1 production, pathogen inhibition and co-aggregation with Candida albicans and Gardnerella vaginalis.
The female lower genital tract is an ecological niche where several microorganisms coexist in a dynamic balance. This ecosystem is dynamic with changes in structure and composition being influenced by age, menarche, time in menstrual cycle, pregnancy, infections, methods of birth control, sexual activity, use of medications and hygiene. In healthy women, the vaginal ecosystem is dominated by Lactobacillus spp. Some of the undesirable organisms are yeasts (Candida albicans, C. Tropicalis, C. krusei), anaerobic bacteria responsible of vaginosis (Gardnerella vaginalis, Atopobium vaginae, Prevotella, Veillonella), uropathogens (E. coli, Proteus, Klebsiella, Serratia), and sexually transmitted viruses (HIV, Herpes virus). Lactobacilli are involved in maintaining the normal vaginal microflora by preventing overgrowth of pathogenic and opportunistic organisms.
The rationale for use of EvaNew vaginal tablet in women is based on the genitourinary regulatory role played by the vaginal microbiota and the need for restoration of this microbial ecosystem after insult. Lactobacilli are commonly used as probiotics. The use of lactobacilli to re-establish a physiological microbial flora of the female urogenital tract dates back to early 1900s. Because antimicrobial treatment of urogenital infections is not always effective, and problems remain due to bacterial and yeast resistance, recurrent infection, as well as side effects, it is not surprising that alternative remedies are of interest to patients and their caregivers.
Mechanism of Action
The principle mechanisms by which EvaNew vaginal tablet exerts its protective functions are (1) stimulation of the local immune system (2) competition with other microorganisms for the nutrients and for adherence to the vaginal epithelium, (3) reduction of the vaginal pH by the production of the organic acids, especially lactic acid, and (4) production of antimicrobial substances, such as bacteriocins and hydrogen peroxide.
The different mechanisms of action of the individual strains are as given below:
- L. brevis possesses strong adherence characteristics and as a virtue of its larger morphology size exerts 1 competitive exclusion of vaginal opportunistic pathogens.
- L. salivarius and L. plantarum produce high amounts of lactic acid maintaining a healthy vaginal pH which is 1 inhibitory to growth of vaginal pathogens including HSV-2 and HIV-1
- Peroxidase, hydrogen peroxide and halides constitute a strong anti-microbial system in phagocytes and in tissue fluids. Peroxidase and halide ions are present in the vaginal fluid while L. salivarius and L. plantarum secrete H2O2 which has anti-bacterial and anti-viral properties. In vitro, hydrogen peroxide producing Lactobacillus strains have a strong inhibitory activity against Gardnerella vaginalis, Prevotella bivia, Neisseria gonorrhoeae, and HIV-1
- The probiotic strains in EvaNew vaginal tablet co-aggregate very efficiently with both Gardnerella vaginalis and Candida albicans, producing a microenvironment around the pathogens, where the concentration of inhibiting substances, produced by lactobacilli, is exacerbated.
- Lactobacillus brevis is rich in arginine deiminase, which uses arginine as a substrate for the production of citrulline, thus preventing its use for the synthesis of polyamines. Arginine deiminase can also act as anti-viral agent suppressing HIV-1 replication in CD4+ cells.
- L. brevis contains high concentration of sphingomyelinase. Sphingomyelin present in the vaginal secretion can be metabolized by sphingomyelinase to generate ceramide. Ceramide inhibits viral infections by membrane-lipid rafts organization and structure. EvaNew tablet therefore may prevent entry of HSV-2 and HIV
- The bacterial strain, L. brevis, in particular has been reported to prevent the binding / entry of HSV-2 in vitro.
5.2 Pharmacokinetic Properties
Lactobacilli are present in most of the dairy products e.g. milk, curd and cheese. In human beings these microorganisms are present in mouth, intestinal tract and vagina. No specific pharmacokinetics data is available for EvaNew vaginal tablet. It acts through local action.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
No known animal toxicology data
7.0 Description
EvaNew vaginal tablet is a mixture of three lactobacilli probiotic strains namely, L. brevis CD2, L. salivarius Fv2 and L. plantarum FV9 which colonize in the vagina and restore a healthy vaginal flora and pH.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not-applicable
8.2 Shelf-life
Please see Mfg. Date/Expiry Date printed on pack. Do not use the product after the expiry date, which is stated on the packaging. The expiry date refers to the last day of that month. The expiry date refers to the product kept unopened in its original packing
8.3 Packaging information
A blister strip of 8 tablets.
8.4 Storage and handing instructions
Store between 2⁰C to 8⁰C. Do not freeze.
Keep out of reach of children.
Any discoloration or spotting on the tablet will not affect its efficacy.
9.0 Patient Counselling Information
- EvaNew vaginal tablets helps to maintain normal vaginal flora and restore healthy vaginal pH, in general, and after clinical treatment of opportunistic infections.
- Advice to the patients: Evanew Vaginal Tablet is inserted into the vagina, preferably at night before going to bed.
- Do not skip any doses and finish the full course of treatment even if you feel better.
- Tell the patients if they get any side effects, talk to the doctor. This includes any possible side effects not listed in this leaflet. Advice patients if they have any further questions, ask the doctor or pharmacist.
12.0 Date of revision of text
02-07-2024
About Leaflet
Read all of this leaflet carefully before your child starts taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for your child. Do not pass it on to others. It may harm them, even if their signs of illness are the same as your child’s.
- If your child gets any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet?
- What EvaNew is and what it is used for?
- What you need to know before you take EvaNew?
- How to take EvaNew?
- Possible side effects
- How to store EvaNew?
- Contents of the pack and other information
1. What EvaNew is and what it is used for?
Evanew is a probiotic vaginal tablet containing a blend of three lactobacilli strains (L. brevis CD2, L. salivarius FV2, and L. plantarum FV9).
Evanew functions by replenishing beneficial probiotics in the vagina, which produce lactic acid to maintain a lower pH. This acidic environment inhibits the growth of harmful bacteria and yeast while the probiotics themselves compete for resources and produce antimicrobial substances like hydrogen peroxide to further protect vaginal health.
It is used to help maintain normal vaginal flora and restore a healthy vaginal pH, for the treatment and prevention of bacterial vaginosis and after clinical treatment of opportunistic infections such as vulvovaginal candidiasis, protozoal, and viral infections.
2. What you need to know before you take EvaNew Tablet?
Do not use EvaNew tablet if you: Do not use if you have a known hypersensitivity to any ingredients listed in section 6.
Warnings and Precautions:
- Do not use if the packaging is broken or damaged.
- Avoid douching during treatment.
- Do not ingest (only for vaginal use).
- Avoid using intra-vaginal antibiotics during treatment.
- Compatible with condoms and other physical barrier methods.
Pregnancy and Breastfeeding
- Evanew vaginal tablets during pregnancy and lactation is considered safe.
- No adverse events have been reported in pregnant women using this product.
- Always talk to your healthcare provider before starting any new medication
Other medicines and EvaNew tablet:
No known drug interaction has been reported yet.
Driving and using machines
EvaNew tablets does not affect your ability to drive or use machines.
3. How to use EvaNew tablet?
Insert 1 tablet intra-vaginally at bedtime for 8 days every month, starting from the 8th day of your menstrual cycle, for 3 consecutive months.
Administration Steps:
- Wash your hands with soap.
- Remove the tablet from the pack.
- Do not break or crush the tablet.
- Insert the tablet inside the vagina while lying down.
If you use more EvaNew tablet than you should:
- If you have used more EvaNew than you should, talk to a doctor
- EvaNew is for local treatment inside the vagina
If you forget to use EvaNew Tablet:
- If you forget a dose, use the medicine as soon as you remember.
- Do not use a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side-effects
- No adverse effects reported on reproductive health and fertility in animals.
- Non-teratogenic.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly:
Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store EvaNew Tablet?
- Store between 2⁰C to 8⁰C.
- Do not freeze.
- Keep out of reach of children.
- Any discoloration or spotting on the tablet will not affect its efficacy.
6. Contents of the pack and other information
A blister strip containing 8 vaginal tablets
Each vaginal tablet contains:
At least 1 Billion CFU of Mix Lactobacilli: L. brevis CD2, L. salivarius FV2 & L. plantarum FV9.
Marketing Authorisation Holder
Zuventus Healthcare Ltd.
Zuventus House, Plot Y2,
CTS No: 358/A2, Near Nahur
Railway Station, Nahur (West),
Mumbai 400 078.
This leaflet was last revised in July 2024.