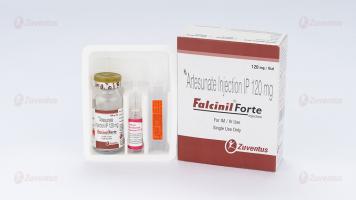
Falcinil & Falcinil Forte Injection
Therapy Area
Anti-malarial
Composition
Each vial contains :
Artesunate IP (Sterile) 60 mg
This pack contains 1 ml ampoule of Sodium Bicarbonate
Injection IP 5% w/v & 5 ml ampoule of Sodium Chloride
Injection IP 0.9% w/v.
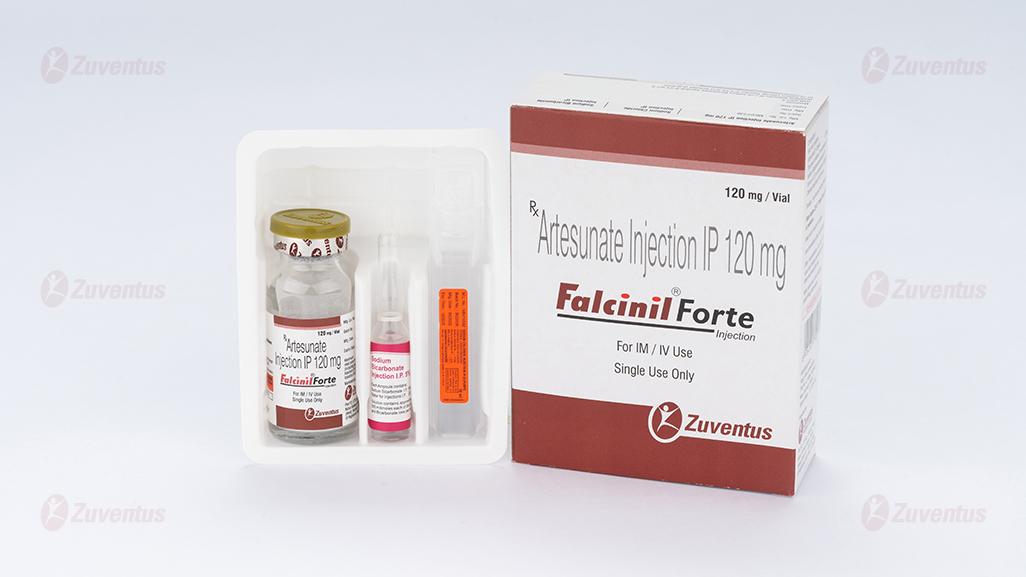
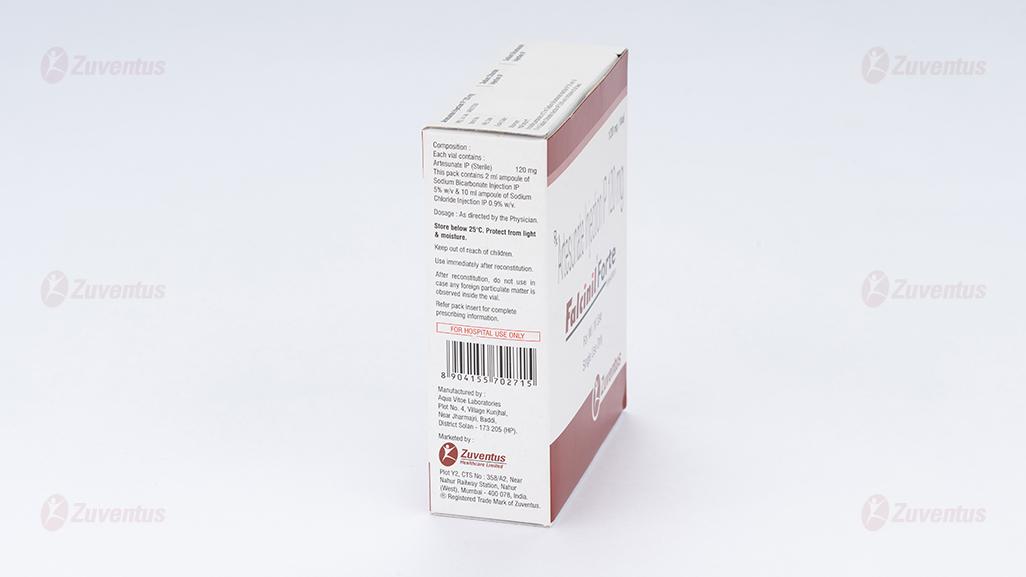
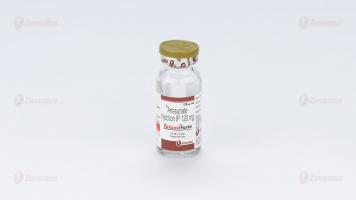
Each vial contains :
Artesunate IP (Sterile) 120 mg
This pack contains 2 ml ampoule of Sodium Bicarbonate
Injection IP 5% w/v & 10 ml ampoule of Sodium Chloride
Injection IP 0.9% w/v.
Description
Chemical formula : C19H28O8
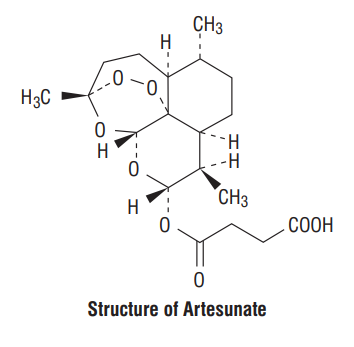
Molecular weight : 384.421 g/mol
Artesunate is the water insoluble hemisuccinate ester of dihydroartemisinin, (Qinghaosu) an anti malarial principle extracted from leaves of the plant Artemisia annua. Artesunate is a white crystalline powder, odorless and almost tasteless. The chemical structure of Artesunate contains a sesquiterpene lactone ring with an endoperoxide bridge. Artesunate is active against multidrug resistant Plasmodium falciparum as well as the sensitive strains. It is also highly active against P. vivax. WHO recommends the use of Artesunate as an injectable preparation for severe malaria.
Clinical Pharmacology
Mechanism of Action
The endoperoxide bridge is essential for the antimalarial activity of Artesunate. This endoperoxide bridge interacts with heme in the parasite. Intra parasitic iron catalyses the cleavage of the endoperoxide bridge of Artesunate within the infected erythrocyte, generating free radicals (singlet oxygen). These highly reactive free radical species binds to membrane proteins, causes lipid peroxidation, damages endoplasmic reticulum, inhibit protein synthesis and ultimately results in lysis of the parasite. Artesunate acts rapidly against asexual erythrocytic stage of plasmodia. Artesunate has gametocytocidal activity but shows no action on hepatic forms (hypnozoites).
Pharmacokinetics
Artesunate is extensively converted to dihydroartemisinin (DHA) by blood esterases.
Falcinil Injection
In patients with severe malaria, the Cmax of DHA ranges from 513 - 5789 nmol/L. The median elimination half-life (t1/2) is 0.34 h (range 0.18 - 0.87 h) and the time to last detectable DHA in blood is around 2 h.
Falcinil Forte Injection
In patients with severe malaria, Artesunate shows a volume of distribution (Vd) of 0.08 liter/kg, clearance (CL) of 1.63 liters/h/kg and a short elimination half-life (t1/2) of 2.3 min. The peak concentration of DHA (Cmax) of 8.5 µmol/liter is reached within 10.4 min (Tmax). The mean AUC of DHA in patients with severe malaria is around 7.3 µmol.h/liter. DHA shows a Vd of 0.77 liter/kg and a t1/2 of 40 min.
Indications
Severe malaria including cerebral malaria.
Contradictions :
Prior hypersensitivity to Artesunate or any other artemisinin derivative
Special Population :
Pediatrics
Parenteral Artesunate is recommended by WHO for severe malaria in children, but it should be used with caution.
Pregnancy
WHO recommends using parenteral Artesunate in severe malaria in patients with second and third trimester pregnancy. Parenteral Artesunate can be considered as an option in first trimester pregnancy by taking into account the risk-benefit ratio.
Lactating mother
Breast feeding should be stopped while using artemisinin derivatives in lactating mothers.
Geriatrics
No special precautions are required when administering parenteral Artesunate to geriatric population.
Patients with renal or hepatic failure
There is no information available regarding the use of Artesunate in patients with renal or hepatic failure. Parenteral Artesunate should be used with caution in such patient population.
Patients with cardiac disorders
Since artemisinin has induced cardio toxic effects in experimental animals and transient first degree block has been documented, it should be used with caution in patients with chronic cardiac disorders.
Dosage :
For adults
Artesunate 2.4 mg/kg body weight; I.V. or I.M. given on admission (time = 0), then at 12 h and 24 h, then once a day until patient is able to tolerate medication when an ACT (artemisinin combination therapy) is given.
For children
<20 kg : 3.0 mg/kg, >20 kg : 2.4 mg/kg; I.V. or I.M. given on admission (time = 0), then at 12 h and 24 h, then once a day until patient is able to tolerate medication when an ACT (artemisinin combination therapy) is given.
Administration :
Falcinil Injection
The powder for injection should be reconstituted with 1 ml of 5% Sodium Bicarbonate Injection IP and Shake vigorously for 5 minutes to get clear solution.
For I.V. use : Add 5 ml of normal saline or 5% w/v Dextrose Injection IP and mix again to prepare final concentration of 10 mg/ml for I.V.
The required amount of drug for I.V. use should be administered slowly over a period of 2 to 3 minutes.
For I.M. use : Add 2 ml of normal saline or 5% w/v Dextrose Injection IP and mix again to prepare final concentration of 20 mg/ml for I.M. use.
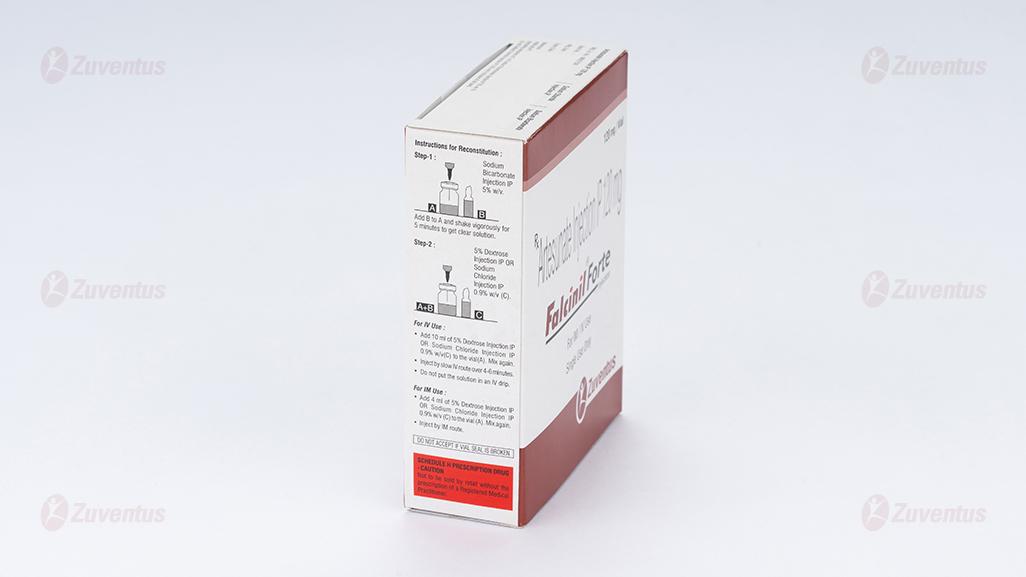
Falcinil Forte Injection
The powder for injection should be reconstituted with 2 ml of 5% Sodium Bicarbonate Injection IP and Shake vigorously for 5 minutes to get clear solution.
For I.V. use : Add 10 ml of normal saline or 5% w/v Dextrose Injection IP and mix again to prepare final concentration of 10 mg/ml for I.V. The required amount of drug for I.V. use should be administered slowly over a period of 4 to 6 minutes
For I.M. use : Add 4 ml of normal saline or 5% w/v Dextrose Injection IP and mix again to prepare final concentration of 20 mg/ml for I.M. use.
- The powder for Injection is difficult to dissolve and care should be taken to ensure that it is completely dissolved before parenteral administration.
- Prepare a fresh solution for each administration.
- The formulation should be used immediately after reconstitution. Discard any unused solution after the use.
- If the solution is cloudy or a precipitate is present, the parenteral solution should be discarded.
Adverse Reactions
Artemisinin and its derivatives appear to be generally well tolerated, although there have been reports of mild gastrointestinal disturbances including nausea, vomiting, headache, tinnitus and neutropenia. Other reported adverse reactions are transient and reversible reticulocytopenia, fever, rash, bradycardia, transient first degree heart block, QT prolongation and transient & reversible elevation of serum transaminases. Evidence of neurotoxicity has been seen in animals when given in high doses.
Drug Interactions
- Artesunate alters the pharmacokinetics of mefloquine, i.e. the peak concentration of mefloquine is reduced and the volume of distribution is expanded.
- Since Artesunate prolonged PR and QT intervals in some experimental animals, some adverse drug interactions could get induced when Artesunate is administered with other antimalarials that have cardiac reactions viz. quinine, quinidine, mefloquine, halofantrine etc.
- Drugs known to prolong QT interval, viz. erythromycin, terfenadine, astemizole, quinidine, tricyclic antidepressants, neuroleptics etc. should not be co-prescribed with Artesunate.
Storage
Store below 25°C. Protect from light & moisture.
Keep out of reach of children.
After reconstitution, do not use in case any foreign particulate matter is observed inside the vial. Use immediately after reconstitution.
Shelf-life
Refer on the pack.
Presentation :
Falcinil Injection
A vial of 60 mg along with 1 ml ampoule of Sodium Bicarbonate Injection IP 5% w/v & 5 ml ampoule of Sodium Chloride Injection IP 0.9% w/v.
Falcinil Forte Injection
A vial of 120 mg along with 2 ml ampoule of Sodium Bicarbonate Injection IP 5% w/v & 10 ml ampoule of Sodium Chloride Injection IP 0.9% w/v.