Falcinil LF Dry Syrup
Therapy Area
Anti-malarial
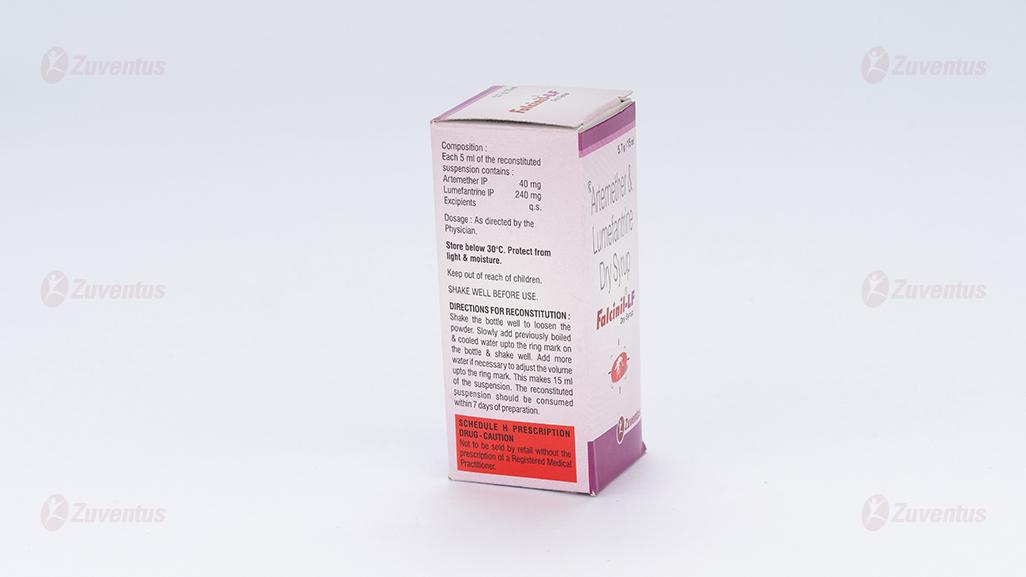
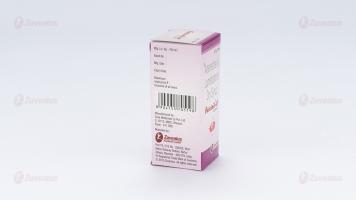
Composition
Each 5 ml of the reconstituted suspension contains :
Artemether IP 40 mg
Lumefantrine IP 240 mg
Excipients q.s.
Description
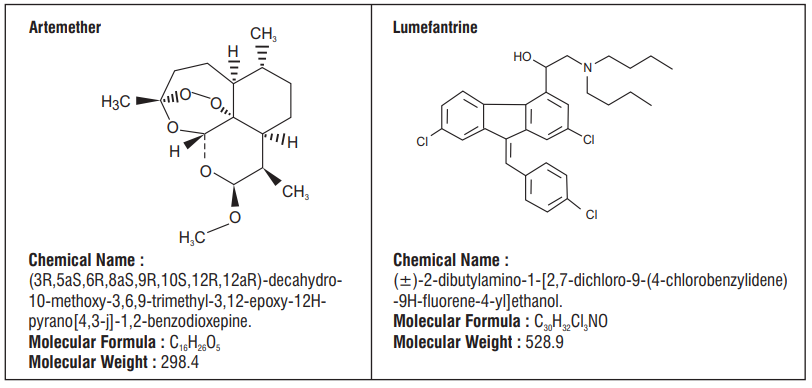
Artemether is a sesquiterpene lactone derived from the naturally occurring substance artemisinin. Lumefantrine is a synthetic racemic fluorine mixture
Clinical Pharmacology
Pharmacodynamics
Falcinil-LF dry syrup comprises a fixed ratio of 1:6 parts of artemether and lumefantrine, respectively. The site of antiparasitic action of both components is the food vacuole of the malarial parasite, where they are thought to interfere with the conversion of heme, a toxic intermediate produced during hemoglobin break down, to the non toxic haemozoin, a malarial pigment. Lumefantrine is thought to interfere with the polymerization process. While artemether generates active metabolites as a result of the interaction between its peroxide bridge and heme iron. Both artemether and lumefantrine have a secondary action involving inhibition of nucleic acid and protein synthesis within the malarial parasite. Data from in vitro and in vivo studies show that Falcinil-LF does not induce resistance.
The independent antimalarial activity of both lumefantrine and artemether is enhanced by their combination into Falcinil-LF, which has been shown to potentiate the blood schizonticidal effects. It is also effective against drug-resistant strains of P. falciparum malaria. Comprehensive in vitro studies using laboratory-maintained and fresh-field parasite isolates from different malaria endemic areas have shown marked synergy of the two components. Results of comparative clinical trials indicate that Falcinil-LF also clears gametocytes more rapidly than non-artemisinin antimalarials.
Activity In Vitro and In Vivo
Artemether and lumefantrine are active against the erythrocytic stages of Plasmodium falciparum.
Absorption
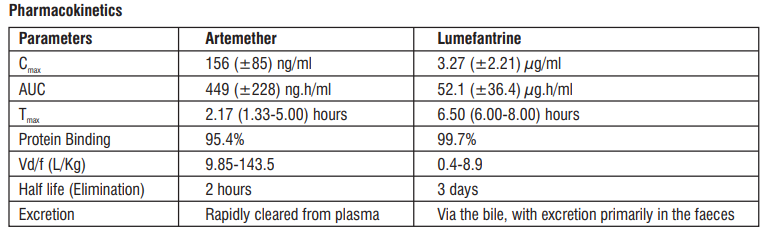
Artemether is absorbed fairly rapidly with peak plasma concentrations reached about 2 hours after dosing. Absorption of lumefantrine, a highly lipophilic compound, starts after a lag-time of up to 2 hours, with peak plasma concentration about 6-8 hours after dosing. Food enhances the absorption of both artemether and lumefantrine in healthy volunteers. The relative bioavailability of artemether was increased more than two-fold and that of lumefantrine sixteen-fold, compared with fasted conditions when Falcinil-LF was taken after a high-fat meal. Food has also been shown to increase the absorption of lumefantrine in patients with malaria, although to lesser extent (approximately two-fold), most probably due to the lower fat content of the food ingested by acutely ill patients. The food interaction data indicate that absorption of lumefantrine under fasted conditions is very poor (assuming 100% absorption after a high-fat meal, the amount absorbed under fasted conditions would be < 10% of the dose). Patients should therefore be encouraged to take the medication with a normal diet as soon as food can be tolerated.
Distribution
Distribution has not been further investigated in humans, but in rats artemether is well distributed throughout the body, with some affinity for the brown fat and adrenal glands, while lumefantrine has an affinity for adipose and glandular tissue and to some extent for the lungs, spleen (due to slow elimination from lymphoid tissue) and bone marrow.
Metabolism
Artemether is rapidly and extensively metabolized (substantial first-pass metabolism) both in vitro and in humans. Human liver microsomes metabolise artemether to the biologically active main metabolite dihydroar temisinin (demethylation), predominantly through the enzymes CYP3A 4/5. This metabolite has also been detected in humans in vivo. Lumefantrine is N-debutylated, mainly by CYP3A4, in human liver microsomes. In vivo in animals (dogs and rats), glucuronidation of lumefantrine takes place directly and after oxidative biotransformation. In vitro lumefantrine significantly inhibits the activity of CYP2D6 at therapeutic plasma concentrations.
Elimination
Artemether is rapidly cleared from plasma with an elimination half-life of about 2 hours. Lumefantrine is eliminated very slowly with a terminal half-life of 2-3 days in healthy volunteers and 4-6 days in patients with falciparum malaria.
Indications
For the treatment of Plasmodium Falciparum malaria cases resistant to both chloroquine and Sulphadoxine – Pyrimethamine combination.
Contraindications
Hypersensitivity to the active substances or to any of the excipients
Dosage in children
Making up the Artemether/lumefantrine oral suspension :
After opening the bottle (breaking the seal), previously boiled & cooled drinking water to make volume 15 ml. After adding the water the mixture is vigorously shaken until all powder has disappeared from the bottom and an oral suspension is being formed. The composition of the powders is such that this process takes only a few seconds. It may be necessary to readjust the volume to the 15 ml mark. This oral suspension is stable for 7 days. It is advisable to shake the bottle before use.
A subunit of 5 ml contains 40 mg Artemether and 240 mg Lumefantrine. Dose should be administered as per body weight as mentioned below in the table. It is recommended to round off the dosage to the nearest subdivision.
Practical scheme for administering the correct dose of Artemether/Lumefantrine oral Suspension :
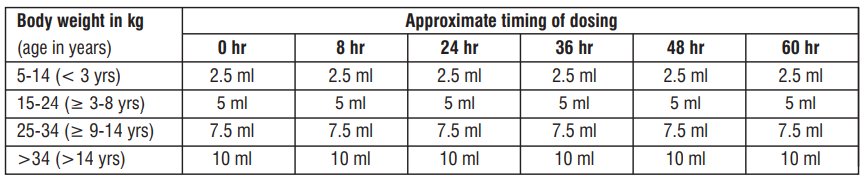
New & recrudescent infections in children
Data for a limited number of patients show that new and recrudescent infections can be treated with a second course of Falcinil-LF. In vitro studies involving samples from patients with recrudescent infection showed no significant decrease in the sensitivity of P. falciparumto either artemether or lumefantrine.
Dosage in patients with renal or hepatic impairment
Although no specific studies have been carried out, no special precautions or dosage adjustments are considered necessary for these conditions. Most patients with acute malaria present with some degree of related hepatic impairment. The adverse event profile did not differ in patients with and those without hepatic impairment. Moreover, baseline abnormalities in liver function tests improved in nearly all patients after treatment with Artemether plus Lumefantrine.
Overdosage
There is no information on overdoses of Falcinil-LF dry syrup, higher than the doses recommended for treatment. In cases of suspected overdosage, symptomatic and supportive therapy, which would include ECG and blood electrolyte monitoring, should be given as appropriate.
Cautions & Precautions :
Falcinil-LF has not been evaluated for the treatment of cerebral malaria or other severe manifestations of complicated malaria including pulmonary oedema or renal failure. Several antimalarials are known to cause QTc-prolongation and a slight QTcprolongation without clinical symptoms has been observed in a few patients treated with Falcinil-LF, mainly in cases where concomitant dehydration or electrolyte imbalance was present. No correlation was found between QTc-prolongation and peak plasma concentration in individual patients. Patients who remain averse to food during treatment should be closely monitored as the risk of recrudescence may be greater.
Drug Interactions :
- CYP3A4 Inducers : Potential for loss of antimalarial efficacy.
- CYP3A4 Inhibitors : Use cautiously due to potential for QT prolongation.
- Anti-Retrovirals : Use cautiously due to potential for QT prolongation, loss of anti-viral efficacy, or loss of antimalarial efficacy of Falcinil-LF dry syrup.
- Mefloquine : If used immediately before treatment, monitor for decreased efficacy of Falcinil-LF dry syrup and encourage food consumption.
- Hormonal Contraceptives : Effectiveness may be reduced; use an additional method of birth control.
- CYP2D6 Substrates (e.g. neuroleptics and tricyclic antidepressants) : Monitor for adverse reactions and potential QT prolongation
Adverse Effects
Steven Johnson Syndrome (SJS) has been seen with Falcinil-LF syrup. The frequency of adverse experience reported in clinical trials of Falcinil-LF in the treatment of malaria was generally similar to or lower than that of other antimalarial drugs used in the clinical trials. Many of the adverse experience observed during clinical testing are to the disease rather than to Falcinil-LF although some symptoms that are of the normal clinical picture of acute malaria may be caused or exacerbated by Falcinil-LF. The most common adverse experiences (> 1%) in patients treated with Falcinil-LF for which causality is suspected are :
Central nervous system : Sleep disorder, headache, dizziness
Cardiovascular system : Palpitation
Gastrointestinal tract : Abdominal pain, anorexia, diarrhoea, vomiting, nausea
Skin and appendages : Pruritus, rash
Respiratory tract : Cough
Musculoskeletal system : Arthralgia, myalgia
Others : Asthenia, fatigue
Incompatibilities:
None known
Storage
Store below 30°C. Protect from light & moisture
Keep out of reach of children.
The reconstituted suspension should be consumed within 7 days of preparation.
Shelf-life
Refer on the pack.