Feronia HP
Therapy Area
Vitamins/Minerals Supplements
1.0 Generic Name
Ferrous ascorbate, Folic Acid, Methylcobalamin and Zinc sulphate
2.0 Qualitative and Quantitative Composition
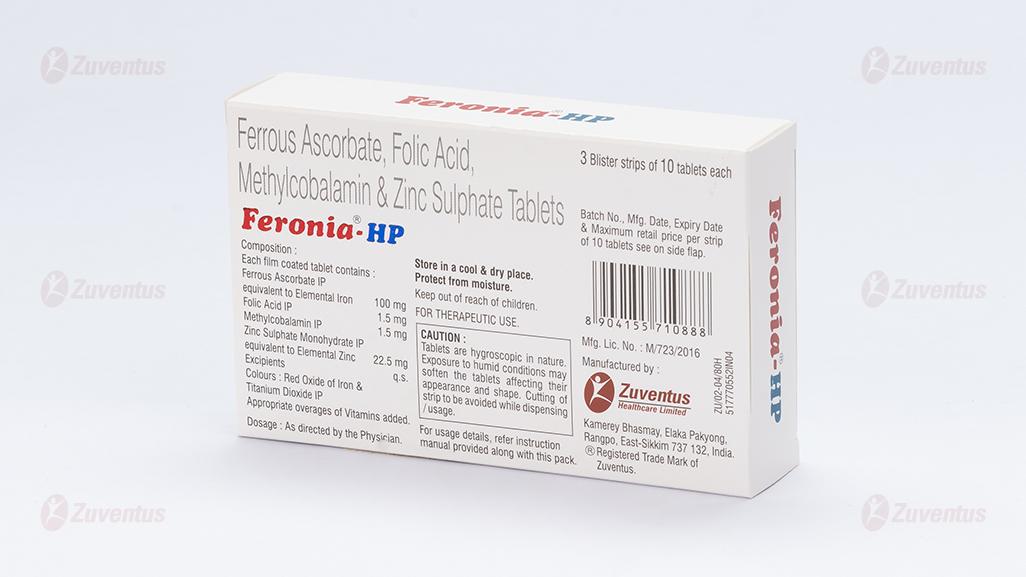
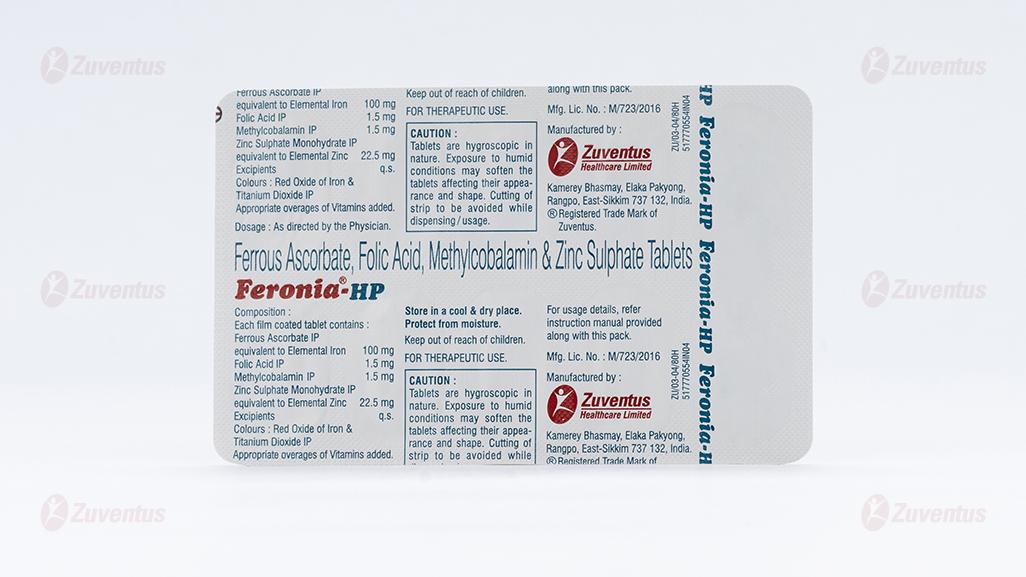
Each Film-coated tablet contains:
Ferrous ascorbate equivalent to Elemental iron 100mg
Folic Acid IP 1.5mg
Methylcobalamin IP 1.5 mg
Zinc sulphate monohydrate eq. 22.5 mg
to Elemental Zinc
Excipients q.s.
Colour: Red Oxide of Iron and Titanium Dioxide IP
3.0 Dosage Form and Strength
Film coated tablet
Ferrous Ascorbate 100 mg, Folic Acid 1.5 mg, Methylcobalamin 1.5 mg, Zinc Sulphate 22.5mg
4.0 Clinical particulars
4.1 Therapeutic indications
For the treatment of iron deficiency anemia.
4.2 Posology and method of administration
Adults
One tablet should be taken once daily by mouth.
Pediatric Population
There is no relevant use of Feronia HP Tablets in the pediatric population.
4.3 Contraindications
- Hypersensitivity to the active substances or any of the excipients.
- Paroxysmal nocturnal hemoglobinuria, hemosiderosis, haemochromatosis, active peptic ulcer, repeated blood transfusion, regional enteritis and ulcerative colitis.
- Feronia HP Tablets must not be used in the treatment of anemias other than those due to iron deficiency.
- Copper deficiency: Zinc may inhibit the absorption of Copper
4.4 Special warnings and precautions for use
CAUTION: Tablets are hygroscopic in nature. Exposure to humid conditions may soften the tablets affecting their appearance and shape. Cutting of strip should be avoided while dispensing/usage.
The label will state “Important warning: Contains Iron. Keep out of reach and sight of children, as overdose may be fatal”.
This will appear on the front of the pack within a rectangle in which there is no other information.
Some post-gastrectomy patients show poor absorption of iron. Care is needed when treating iron deficiency anemia in patients with treated or controlled peptic ulceration. Caution should be exercised when administering folic acid to patients who may have folate dependent tumors.
Since anemia due to combined iron and vitamin B12 or folate deficiencies may be microcytic in type, patients with microcytic anemia resistant to therapy with iron alone should be screened for vitamin B12 or folate deficiency.
Accumulation of zinc may occur in cases of renal failure.
Pregnancy
Safety is not established in pregnant women; therefore Feronia HP is not recommended in pregnancy.
Lactation
Safety is not established in lactating women; therefore Feronia HP is not recommended during lactation.
Pediatric population
Feronia HP Tablets should be kept out of the reach of children.
4.5 Interaction with other medicinal products and other forms of interaction
- Iron reduces the absorption of penicillamine. Iron compounds impair the bioavailability of fluoroquinolones, levodopa, carbidopa, thyroxine and bisphosphonates.
- Absorption of both iron and antibiotic may be reduced if Feronia HP is given with tetracycline.
- Concurrent administration of antacids may reduce absorption of iron. Co- trimoxazole, chloramphenicol, sulphasalazine, aminopterin, methotrexate, pyrimethamine or sulphonamides may interfere with folate metabolism. Serum levels of anticonvulsant drugs may be reduced by administration of folate.
- Oral chloramphenicol delays plasma iron clearance, incorporation of iron into red blood cells and interferes with erythropoiesis.
- Some inhibition of iron absorption may occur if it is taken with cholestyramine, trientine, tea, eggs or milk.
- Administration of oral iron may increase blood pressure in patients receiving methyldopa.
- Coffee may be a factor in reducing iron bioavailability. Neomycin may alter the absorption of iron.
- The absorption of zinc may be reduced by calcium supplements, tetracyclines and phosphorus-containing compounds, agents which increase gastric pH, such as H2 blockers, may decrease zinc absorption while zinc may reduce the absorption of penicillamine, tetracyclines, fluoroquinolones.
Food
Studies of the co-administration of zinc with food performed in healthy volunteers showed that the absorption of zinc was significantly delayed by many foods (including bread, hard boiled eggs, coffee and milk). Substances in food, especially phytates and fibres, bind zinc and prevent it from entering the intestinal cells. However, protein appears to interfere the least.
4.6 Fertility, pregnancy and lactation
Pregnancy
Safety is not established in pregnant women; therefore Feronia HP is not recommended in pregnancy. Breast-feeding Safety is not established in lactating women; therefore Feronia HP is not recommended during lactation. Fertility No fertility data is available.
4.7 Effects on ability to drive and use machines
Feronia HP Tablets has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Very rare (<1/10,000)
Very rare (<1/10,000):
Rarely allergic reactions may occur.
Not known (cannot be estimated from the available data)
Not known: Gastrointestinal disorders Gastro-intestinal discomfort, anorexia, nausea, vomiting, constipation, diarrhea.
Not known: Renal and urinary disorders Darkening of the stools may occur. Blood amylase, lipase and alkaline phosphatase increased
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx
4.9 Overdose
Symptoms
Symptoms and signs of abdominal pain, vomiting and diarrhea appear within 60 minutes. Cardiovascular collapse with coma may follow. Some improvement may occur after this phase which, in some patients, is followed by recovery. In others, after about 16 hours, deterioration may occur involving diffuse vascular congestion, pulmonary edema, convulsions, anuria, hypothermia, severe shock, metabolic acidosis, coagulation abnormalities and hypoglycemia.
Management
Vomiting should be induced immediately, followed as soon as possible by parenteral injection of desferrioxamine mesylate, and then gastric lavage. In the meantime, it is helpful to give milk and/or 5% sodium bicarbonate solution by mouth.
Dissolve 2g desferrioxamine mesylate in 2 to 3ml of water for injections and give intramuscularly. A solution of 5g desferrioxamine in 50 to 100ml of fluid may be left in the stomach. If desferrioxamine is not available, leave 300ml of 1 % to 5 % sodium bicarbonate in the stomach. Fluid replacement is essential.
Recovery may be complicated by long-term sequelae such as hepatic necrosis, pyloric stenosis or acute toxic encephalitis which may lead to CNS damage.
Treatment of overdose should be with gastric lavage or induced emesis as quickly as possible to remove unabsorbed zinc. Heavy metal chelation therapy should be considered if plasma zinc levels are markedly elevated (> 10 mg/l).
Pediatric population
Acute overdose of oral iron requires emergency treatment. In young children 200-250mg/kg Ferrous Ascorbate is considered to be extremely dangerous.
5.0 Pharmacological properties
5.1 Mechanism of Action/ Pharmacodynamic properties
Iron absorption occurs predominantly in the duodenum and upper jejunum. Iron is oxidized to the Fe3+ state no matter its original form when taken in orally. Gastric acidity as well as solubilizing agents such as ascorbate prevent precipitation of the normally insoluble Fe3+. Intestinal mucosal cells in the duodenum and upper jejunum absorb the iron. The iron is coupled to transferrin (Tf) in the circulation which delivers it to the cells of the body. A feedback mechanism exists that enhances iron absorption in people who are iron deficient. In contrast, people with iron overload dampen iron absorption. A number of dietary factors influence iron absorption. Ascorbate increase iron uptake in part by acting as weak chelators to help to solubilize the metal in the duodenum. Iron is readily transferred from these compounds into the mucosal lining cells.
Zinc supplementation improves immunity.
Zinc deficiency also has direct effects on the gastrointestinal tract such as impaired intestinal brush border, increased secretion in response to bacterial enterotoxins and perturbations in intestinal permeability. Zinc supplementation improves the transport of water and electrolytes across the intestinal mucosa in experimental zinc deficiency.
Zinc plays a fundamental role in cellular metabolism and is postulated to modulate host resistance to various infections.
5.2 Pharmacokinetic properties
Absorption
Iron is absorbed chiefly in the duodenum and jejunum. Folic Acid is absorbed mainly from the proximal part of the small intestine.
Distribution
The amounts of Folic Acid absorbed from normal diets are rapidly distributed in body tissues.
Biotransformation
Absorption being aided by the acid secretion of the stomach and being more readily affected when the iron is in the ferrous state.
Folic acid rapidly appears in the blood, where it is extensively bound to plasma proteins.
When larger amounts are absorbed, a high proportion is metabolized in the liver to other active forms of folate and a proportion is stored as reduced and methylated folate.
Elimination
Larger amounts of folate are rapidly excreted in the urine and about 4 to 5 micrograms is excreted in the urine daily.
Zinc is absorbed in the small intestine and its absorption kinetics suggest a tendency to saturation at increasing doses. Fractional zinc absorption is negatively correlated with zinc intake. It ranges from 30 to 60% with usual dietary intake (7-15 mg/d) and decreases to 7% with pharmacological doses of 100 mg/d.
In the blood, about 80% of absorbed zinc is distributed to erythrocytes, with most of the remainder being bound to albumin and other plasma proteins. The liver is the main storage for zinc and hepatic zinc levels are increased during maintenance therapy with zinc. The plasma elimination half-life of zinc in healthy subjects is around 1 hour after a dose of 45 mg. The elimination of zinc results primarily from fecal excretion with relatively little from urine and sweat. The fecal excretion is in the greatest part due to the passage of unabsorbed zinc but it is also due to endogenous intestinal secretion.
6.0. Description
This Product (tablet) contains: Ferrous Ascorbate, Folic Acid, Methylcobalamin and Zinc sulphate as active ingredients, for the treatment of iron deficiency anemia.
Ferrous Ascorbate is an iron supplement used to treat or prevent low blood levels of iron (such as those caused by anemia or during pregnancy). Ascorbic acid (vitamin C) improves the absorption of iron from the stomach.
Molecular Formula- C12H14FeO12 Molecular Weight: 406.08g/mol

Folic acid is used to treat anemia caused by folate deficiency. Folic acid is also used as a supplement by women during pregnancy to reduce the risk of neural tube defects (NTDs) in the baby.
Molecular Formula-C19H19N7O6
Molecular Weight- 441.4 g/mol

Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. Zinc is also an essential nutrient element for coral growth as it is an important cofactor for many enzymes.

Molecular Formula-ZnSO4
Molecular Weight- 179.47 g/mol
Methylcobalamin a form of vitamin B12.
Methylcobalamin is equivalent physiologically to vitamin B12, and can be used to prevent or treat pathology arising from a lack of vitamin B12 intake. Methylcobalamin is also used in the treatment of peripheral neuropathy, diabetic neuropathy, and as a preliminary treatment for amyotrophic lateral sclerosis.

Molecular formula: C63H91CoN13O14P
Molecular weight: 1344.405 g·mol−1
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
3 blister strips of 10 tablets each
8.4 Storage and handing instructions
Store at a temperature not exceeding 25°C. Protect from light.
Keep out of reach of children.
9.0 Patient Counselling Information
Patient Counselling Information
- WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor immediately.
- Feronia HP Tablet is given to fulfil your nutritional requirement and to prevent any related diseases.
- Avoid taking antacids 2 hours before or after taking Feronia HP Tablet as they may make it harder for your body to absorb the medicine.
- Let your doctor know if you are taking any other medications like antihypertensive, antibiotics, or medicines for heart disease or bone disorders.
12.0 Date of revision
Sept 2024
Read this entire leaflet carefully before you start taking this medicine because it contains important information for you
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
1. What Feronia HP Tablets is and what it is used for?
Feronia HP film-coated Tablets (referred to as Feronia HP in this leaflet) contains Ferrous Ascorbate 100 mg, Folic Acid 1.5 mg, Methylcobalamin 1.5 mg, Zinc Sulphate 22.5mg. These work together in the medicine.
Feronia HP belongs to a group of medicines called haematinics and vitamin, mineral supplement.
Feronia HP works as a supplement. It provides the body with more iron and folic acid. These are important substances that your body needs to form red blood cells. If you do not have the right amount of these substances, it is possible that you may develop anemia.
Feronia HP is used to prevent and treat low levels of iron and folic acid in the blood (such as those caused by anemia or during pregnancy). Ascorbic acid (vitamin C) improves the absorption of iron from the stomach. Zinc supplementation improves immunity. Methylcobalamin is a form of Vitamin B12, important for the brain and nerves and for the production of red blood cells.
2. What you need to know before you take Feronia HP Tablets?
Do not take Feronia HP:
- if you are allergic to Ferrous Ascorbate and Folic Acid or any of the other ingredients of this medicine
- if you are breast-feeding or trying to become pregnant
- if you suffer from a blood disorder
- if you have had or are having repeated blood transfusions
- if you have a stomach ulcer or other digestive conditions such as regional enteritis or ulcerative colitis
- if you are suffering from anemia that is not due to a lack of iron
If any of the above applies to you, talk to your doctor or pharmacist.
Warnings and precautions
Talk to your doctor before taking Feronia HP
- if you have been or you are being treated for a stomach ulcer
- if you have had or you have a folate dependent tumour
- if you have had all or part of your stomach removed
Feronia HP contains iron. Keep out of reach and sight of children, as overdose may be fatal.
Children
There is no relevant use of Feronia HP in children.
Other medicines and Feronia HP
Tell your doctor if you are taking any other medicines.
- Antibiotics e.g. fluoroquinolones, cotrimoxazole, chloramphenicol, sulphonamides, tetracyclines, neomycin (used for infections)
- Anticonvulsant medicines (used for epilepsy)
- Antacids
- Penicillamine (used for rheumatoid arthritis)
- Sulfasalazine (used for rheumatoid arthritis and bowel disease, e.g. Crohn’s disease)
- Cholestyramine (used for reducing blood cholesterol or control diarrhea)
- Levodopa or Carbidopa (used for Parkinson’s disease)
- Thyroxine (used for thyroid disease)
- Bisphosphonates (used for bone disease)
- Aminopterin and Methotrexate (used for certain cancers)
- Pyrimethamine (used for malaria)
- Trientine (used for Wilson’s disease)
- Methyldopa (used for high blood pressure)
- Any other medicine, including medicines obtained without a prescription
Feronia HP with food and drink
If you drink tea, coffee or milk or eat eggs at the same time as taking Feronia HP, your body may absorb less of the iron and zinc supplement, which may reduce the effect of this medicine.
Pregnancy and breast-feeding
If you are pregnant or think you may be pregnant, ask your doctor for advice before taking this medicine.
Driving and using machines.
There are no known effects on driving or using machines.
3. How to take Feronia HP Tablets?
Always take Feronia HP Tablets exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The recommended dose for adults
- The usual dose is one tablet each day to be taken by mouth.
Method of administration: For oral administration only.
Swallow the tablet whole with a glass of water.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek immediate medical help if you have an allergic reaction.
This includes any of the following symptoms:
- Difficulties in breathing
- Swelling of your eyelids, face or lips
- Rash or itching
Not known: frequency cannot be estimated from the available data
- Upset stomach
- Anorexia (e.g. loss of appetite)
- Sickness
- Constipation
- Diarrhea
- Darkening of stools
Reporting of side effects: If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.You can also report side effects directly: Website: www.zuventus.com in and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.co.in/drug-safety-reporting
5. How should I store Feronia HP Tablets?
- Keep this medicine out of the sight and reach of children. An overdose can be fatal.
- Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of that month.
- Store below 25°C. Keep the blister in the outer carton in order to protect from light.
- Do not throw away any medicines via wastewater or household waste.
- Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
- Ferrous Ascorbate 100 mg
- Folic Acid 1.5 mg
- Methylcobalamin 1.5 mg
- Zinc Sulphate 22.5mg
Pack size:
3 blister strips of 10 tablets each