Feronia IV 50
Therapy Area
Vitamins/Minerals Supplements
1.0 Generic Name
Iron Sucrose Injection USP
2.0 Qualitative and quantitative composition
Feronia-IV 50
Each ml contains: Ferric Hydroxide in Complex with Sucrose
equivalent to Elemental Iron……………………20 mg
Feronia-IV 100
Each ml contains:
Ferric Hydroxide in Complex with Sucrose
equivalent to Elemental Iron…………………. 20 mg
3.0 Dosage form and strength
Solution for injection or infusion.
50mg/2.5 ml ampoule
100mg/5 ml ampoule
4.0 Clinical particulars
4.1. Therapeutic indications
1. Treatment of iron deficiency anemia in patients with chronic kidney disease (CKD).
2. Treatment of iron deficiency in the following indications:
- Where there is a clinical need to deliver iron rapidly to iron stores,
- In patients who cannot tolerate oral iron therapy or who are non-compliant,
- In active inflammatory bowel disease where oral iron preparations are ineffective. The diagnosis of iron deficiency must be based on appropriate laboratory tests (e.g. Hb, serum ferritin, transferrin saturation, serum iron, etc.).
4.2. Posology and method of administration
Monitor carefully patients for signs and symptoms of hypersensitivity reactions during and following each administration of Feronia-IV.
Feronia-IV should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where
full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each Feronia-IV injection. Administration: Feronia-IV must only be administered by the intravenous route. This may be by a slow intravenous injection or by an intravenous drip infusion. Feronia-IV must not be used for intramuscular injection.
Adults and the elderly: The total cumulative dose of Feronia-IV, equivalent to the total iron deficit (mg), is determined by the haemoglobin level and body weight.
The dose for Feronia-IV must be individually determined for each patient according to the total iron deficit calculated with the following formula: Dosage Calculation:
Dose= (Body Weight) x (Target Hb in gm % - Actual Hb in gm %) x 2.4 + Depot iron mg.
- Below 35 kg body weight: target Hb = 13 gm % and depot iron = 15 mg/kg body weight
- 35 kg body weight and above: target Hb = 15 gm% and depot iron = 500 mg
The total amount of Feronia-IV required in mg is determined from above calculation.
Alternatively, the total amount of Feronia-IV required in ml is determined from the following formula or dosage table.

Treatment of iron deficiency
Chronic Iron Deficiency Anemia:
Dosage Calculation: Body Weight X (Target Hb in gm % - Actual Hb in gm %) x 2.4 + 500mg for iron store.
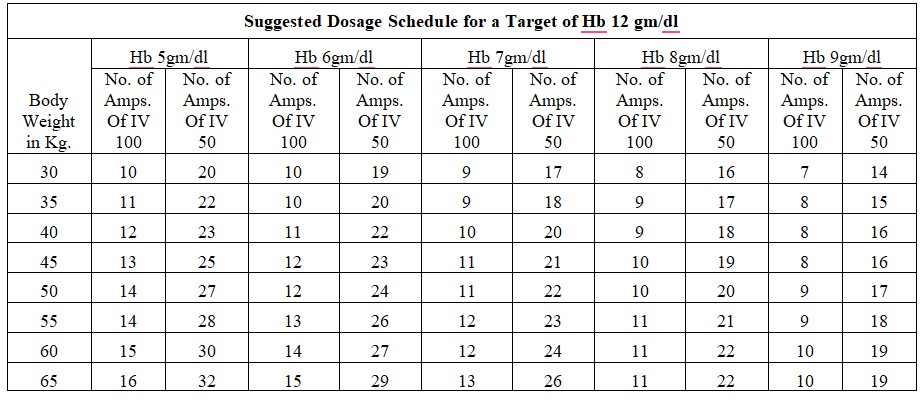

Acute Iron Deficiency Anemia:
Dosage Calculation: Body Weight x (Target Hb in gm % - Actual Hb in gm %)x 2.4
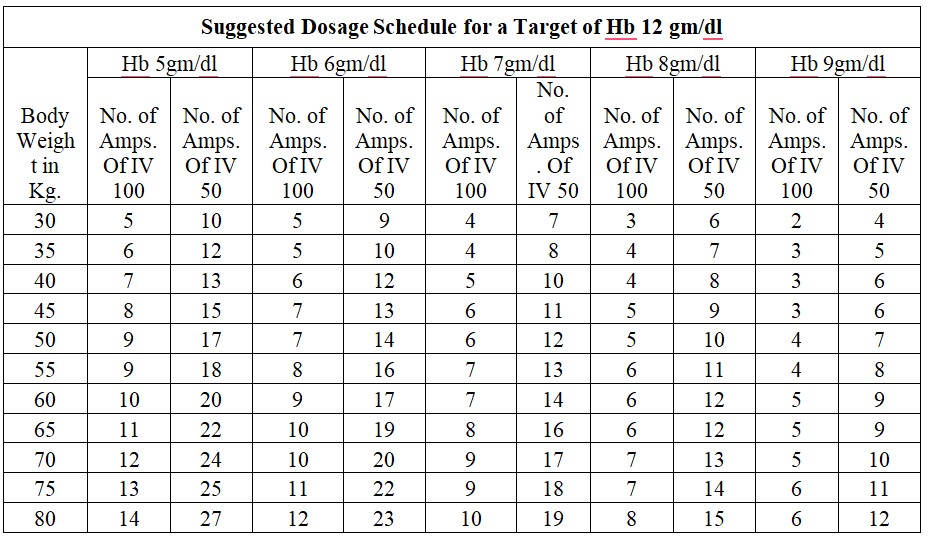
Dosage: The total single dose must not exceed 200 mg of iron given not more than three times per week. If the total necessary, dose exceeds the maximum allowed single dose, then the administration has to be split.
Intravenous drip infusion: Feronia-IV must be diluted only in sterile 0.9% m/V sodium chloride solution:
- 2.5 ml Feronia-IV (50 mg iron) in max. 50 ml sterile 0.9% m/V sodium chloride solution
- 5 ml Feronia-IV (100 mg iron) in max. 100 ml sterile 0.9% m/V sodium chloride solution
- 10 ml Feronia-IV (200 mg iron) in max. 200 ml sterile 0.9% m/V sodium chloride solution
For stability reasons, dilutions to lower Feronia-IV concentrations are not permissible.
Dilution must take place immediately prior to infusion and the solution should be administered as follows:
- 100 mg iron (5 ml Feronia-IV) in at least 15 minutes
- 200 mg iron (10 ml Feronia-IV) in at least 30 minutes
Intravenous injection: Feronia-IV may be administered by slow intravenous injection at a rate of 1 ml undiluted solution per minute and not exceeding 10 ml Feronia-IV (200 mg iron) per injection.
Injection into dialyser: Feronia-IV may be administered during a haemodialysis session directly into the venous limb of the dialyser under the same procedures as those outlined for intravenous injection.
Pediatric Patients
Recommended Treatment Regime in Pediatric Patients with Iron Deficiency Anemia
Using Iron Sucrose
1. Total cumulative dose (mg) = [Target Hb - Actual Hb] x weight (kg) x 2.4 + [15 x weight (kg)], Hb in g/dl.
Calculation of total iron deficit for initial repletion:
2. Dosing:
- Divide calculated total cumulate dose and give every 3-7 days until total dose is administered.
- Recommended maximum single dose is 300 mg or 7 mg iron/kg to prevent adverse effects
3. Administration:
Children (>1 month of age)
Dilute doses ≤ 100 mg 1:1 with NS. Infuse over 30 min
Dilute 200 mg in 200 ml NS. Infuse over 1 hr
Dilute 300 in 250 ml NS. Infuse over at least 1½ hr
NOTE: Do not mix Feronia - IV with other medications or add to parenteral nutrition solutions for intravenous infusion. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
Adult Patients with Hemodialysis Dependent-Chronic Kidney Disease (HDD-CKD)
Administer Feronia-IV 100 mg undiluted as a slow intravenous injection over 2 to 5 minutes, or as an infusion of 100 mg diluted in a maximum of 100 mL of 0.9% NaCl over a period of at least 15 minutes, per consecutive hemodialysis session. Feronia-IV should be administered early during the dialysis session. The usual total treatment course of Feronia-IV is 1000 mg. Feronia-IV treatment may be repeated if iron deficiency reoccurs.
Adult Patients with Non-Dialysis Dependent-Chronic Kidney Disease (NDD-CKD)
Administer Feronia-IV 200 mg undiluted as a slow intravenous injection over 2 to 5 minutes or as an infusion of 200 mg in a maximum of 100 mL of 0.9% NaCl over a period of 15 minutes. Administer on 5 different occasions over a 14-day period. There is limited experience with administration of an infusion of 500 mg of Feronia-IV, diluted in a maximum of 250 mL of 0.9% NaCl, over a period of 3.5 to 4 hours on Day 1 and Day 14. Feronia-IV treatment may be repeated if iron deficiency reoccurs. Adult Patients with Peritoneal Dialysis Dependent-Chronic Kidney Disease (PDD-CKD)
Administer Feronia-IV in 3 divided doses, given by slow intravenous infusion, within a 28 day period: 2 infusions each of 300 mg over 1.5 hours 14 days apart followed by one 400 mg infusion over 2.5 hours 14 days later. Dilute Feronia-IV in a maximum of 250 mL of 0.9% NaCl. Feronia-IV treatment may be repeated if iron deficiency reoccurs.
Pediatric Patients (2 years of age and older) with HDD-CKD for iron maintenance treatment
The dosing for iron replacement treatment in pediatric patients with HDD-CKD has not been established. For iron maintenance treatment: Administer Feronia-IV at a dose of 0.5 mg/kg, not to exceed 100 mg per dose, every two weeks for 12 weeks given undiluted by slow intravenous injection over 5 minutes or diluted in 25 mL of 0.9% NaCl and administered over 5 to 60 minutes. Feronia-IV treatment may be repeated if necessary.
Pediatric Patients (2 years of age and older) with NDD-CKD or PDD-CKD who are on erythropoietin therapy for iron maintenance treatment
The dosing for iron replacement treatment in pediatric patients with NDD-CKD or PDD-CKD has not been established.
For iron maintenance treatment: Administer Feronia-IV at a dose of 0.5 mg/kg, not to exceed 100 mg per dose, every four weeks for 12 weeks given undiluted by slow intravenous injection over 5 minutes or diluted in 25 mL of 0.9% NaCl and administered over 5 to 60 minutes. Feronia-IV treatment may be repeated if necessary.
4.3. Contraindications
The use of Feronia IV is contraindicated in the following conditions:
- Hypersensitivity to the active substance, to Feronia IV or any of its excipients
- Known serious hypersensitivity to other parenteral iron products
- Anaemia not caused by iron deficiency
- Evidence of iron overload or hereditary disturbances in utilisation of iron.
4.4.Special warnings and precautions for use
Parenterally administered iron preparations can cause hypersensitivity reactions including serious and potentially fatal anaphylactic/anaphylactoid reactions. Hypersensitivity reactions have also been reported after previously uneventful doses of
parenteral iron complexes.
The risk is enhanced for patients with known allergies including drug allergies, including patients with a history of severe asthma, eczema or other atopic allergy. There is also an increased risk of hypersensitivity reactions to parenteral iron complexes in patients with immune or inflammatory conditions (e.g. systemic lupus erythematosus, rheumatoid arthritis).
Feronia-IV should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. Each patient should be observed for adverse effects for at least 30 minutes following each Feronia-IV injection. If hypersensitivity reactions or signs of intolerance occur during administration, the treatment must be stopped immediately. Facilities for cardio respirator y resuscitation and equipment for handling acute anaphylactic/anaphylactoid reactions should be available, including an injectable 1:1000 adrenaline solution. Additional treatment with antihistamines and/or corticosteroids should be given as appropriate.
In patients with liver dysfunction, parenteral iron should only be administered after careful risk/benefit assessment. Parenteral iron administration should be avoided in patients with hepatic dysfunction where iron overload is a precipitating factor, in particular Porphyria Cutanea Tarda (PCT). Careful monitoring of iron status is recommended to avoid iron overload.
Parenteral iron must be used with caution in case of acute or chronic infection. It is recommended that the administration of iron sucrose is stopped in patients with ongoing bacteraemia. In patients with chronic infection a risk/benefit evaluation has to be performed, taking into account the suppression of erythropoiesis.
Hypotensive episodes may occur if the injection is administered too rapidly. Allergic reactions, sometimes involving arthralgia, have been more commonly observed when the recommended dose is exceeded.
Paravenous leakage must be avoided because leakage of Feronia-IV at the injection site may lead to pain, inflammation, tissue necrosis and brown discoloration of the skin.
4.5.Interaction with other medicinal products and other forms of interaction
As with all parenteral iron preparations, Feronia-IV should not be administered concomitantly with oral iron preparations since the absorption of oral iron is reduced. Therefore, oral iron therapy should be started at least 5 days after the last injection of Feronia-IV.
4.6.Fertility, pregnancy and lactation
There are no adequate and well-controlled trials of iron sucrose in pregnant women. A careful risk/benefit evaluation is therefore required before use during pregnancy and Feronia-IV should not be used during pregnancy unless clearly necessary.
Iron deficiency anaemia occurring in the first trimester of pregnancy can in many cases be treated with oral iron. Treatment with Feronia-IV should be confined to second and third trimester if the benefit is judged to outweigh the potential risk for both the mother and the foetus.
Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development. Data on a limited number of exposed human pregnancies indicated no adverse effects of Feronia-IV on pregnancy or on the health of the foetus/newborn child. Non metabolised Feronia-IV is unlikely to pass into the mother's milk. No well-controlled clinical studies are available to date. Animal studies do not indicate direct or indirect harmful effects to the nursing child.
4.7.Effects on ability to drive and use machines
In the case of symptoms of dizziness, confusion or light headedness following the administration of Feronia IV, patients should not drive or use machinery until the symptoms have ceased.
4.8.Undesirable effects
The most frequently reported adverse drug reactions (ADRs) of iron sucrose in clinical trials were transient taste perversion, hypotension, fever and shivering, injection site reactions and nausea, occurring in 0.5 to 1.5% of the patients. Non-serious anaphylactoid reactions occurred rarely. In general, anaphylactoid reactions are potentially the most serious adverse reactions.
In clinical trials, the following adverse drug reactions have been reported in temporal relationship with the administration of iron sucrose, with at least a possible causal relationship:
Nervous system disorders
Common (≥ 1/100, < 1/10): transient taste perversions (in particular metallic taste). Uncommon (≥1/1000, < 1/100): headache, dizziness. Rare (≥1/10000, < 1/1000): paraesthesia, syncope, loss of consciousness, burning sensation.
Cardio-vascular disorders
Uncommon (≥ 1/1000, < 1/100): hypotension and collapse, tachycardia and palpitations.
Rare (≥1/10000, < 1/1000): hypertension.
Respiratory, thoracic and mediastinal disorders
Uncommon (≥1/1000, < 1/100): bronchospasm, dyspnoea.
Gastrointestinal disorders
Uncommon (≥1/1000, < 1/100): nausea; vomiting, abdominal pain, diarrhoea.
Skin and subcutaneous tissue disorders
Uncommon (≥ 1/1000, < 1/100): pruritus, urticaria, rash, exanthema, erythema.
Musculoskeletal, connective tissue and bone disorders
Uncommon (≥ 1/1000, < 1/100): muscle cramps, myalgia.
General disorders and administration site disorders
Uncommon (≥ 1/1000, < 1/100): fever, shivering, flushing, chest pain and tightness. Injection site disorders such as superficial phlebitis, burning, swelling. Rare (≥ 1/10000, < 1/1000): arthralgia, peripheral oedema, fatigue, asthenia, malaise, feeling hot, oedema.
Immune system disorders
Rare (≥ 1/10000, < 1/1000): anaphylactoid reactions. Moreover, in spontaneous reports the following adverse reactions have been reported: Isolated cases: reduced level of consciousness, light-headed feeling, confusion, angio-oedema, swelling of joints, hyperhidrosis, back pain, bradycardia, chromaturia.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions
via email to: medico@zuventus.com
Website: Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Overdosage can cause acute iron overloading which may manifest itself as haemosiderosis. Overdosage should be treated, if required, with an iron chelating agent.
5.0 Pharmacological properties
5.1 Pharmacodynamic properties
Mechanism of Action
Iron sucrose, the active ingredient of Feronia-IV, is composed of a polynuclear iron(III)-hydroxide core surrounded by a large number of non-covalently bound sucrose molecules. The complex has a weight average molecular weight (Mw) of approximately 43 kDa. The polynuclear iron core has a structure similar to that of the core of the physiological iron storage protein ferritin. The complex is designed to provide, in a controlled manner, utilisable iron for the iron transport and storage proteins in the body (i.e., transferrin and ferritin, respectively).
Following intravenous administration, the polynuclear iron core from the complex is taken up predominantly by the reticuloendothelial system in the liver, spleen, and bone marrow. In a second step, the iron is used for the synthesis of Hb, myoglobin and other iron-containing enzymes, or stored primarily in the liver in the form of ferritin.
5.2 Pharmacokinetic properties
In healthy adults administered intravenous doses of iron sucrose, its iron component exhibited first order kinetics with an elimination half-life of 6 h, total clearance of 1.2 L/h, and steady state apparent volume of distribution of 7.9 L. The iron component appeared to distribute mainly in blood and to some extent in extravascular fluid. A study evaluating iron sucrose containing 100 mg of iron labelled with 52Fe/59Fe in patients with iron deficiency showed that a significant amount of the administered iron is distributed to the liver, spleen and bone marrow and that the bone marrow is an irreversible iron trapping compartment. Following intravenous administration of Feronia-IV, iron sucrose is dissociated into iron and sucrose. The sucrose component is eliminated mainly by urinary excretion. In a study evaluating a single intravenous dose of iron sucrose containing 1,510 mg of sucrose and 100 mg of iron in 12 healthy adults (9 females, 3 male: age range 32 to 52), 68.3% of the sucrose was eliminated in urine in 4 h and 75.4% in 24 h. Some iron was also eliminated in the urine. Neither transferrin nor transferrin receptor levels changed immediately after the dose administration. In this study and another study evaluating a single intravenous dose of iron sucrose containing 500 to 700 mg of iron in 26 patients with anemia on erythropoietin therapy (23 females, 3 male; age range 16 to 60), approximately 5% of the iron was eliminated in urine in 24 h at each dose level. The effects of age and gender on the pharmacokinetics of iron sucrose have not been studied.
Pharmacokinetics in Pediatric Patients
In a single-dose PK study of Feronia-IV, patients with NDD-CDK ages 12 to 16 (N=11) received intravenous bolus doses of Feronia-IV at 7 mg/kg (maximum 200 mg) administered over 5 minutes. Following single dose Feronia-IV, the half-life of total serum iron was 8 hours. The mean Cmax and AUC values were 8545 μg/dl and 31305 hr•μg/dL, respectively, which were 1.42- and 1.67-fold higher than dose adjusted adult Cmax and AUC values.
Feronia-IV is not dialyzable through CA210 (Baxter) High Efficiency or Fresenius F80A High Flux dialysis membranes. In in vitro studies, the amount of iron sucrose in the dialysate fluid was below the levels of detection of the assay (less than 2 parts per million).
6.0 Nonclinical properties
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated dose toxicity, genotoxicity and toxicity to reproduction and development.
7.0 Description
Therapeutic class: Hematinic
Feronia-IV (iron sucrose injection, USP) is a brown, sterile, aqueous, complex of polynuclear iron (III)- hydroxide in sucrose for intravenous use.
Iron sucrose injection has a molecular weight of approximately 34,000 – 60,000 daltons
Structural formula: [Na2Fe5O8(OH) ⋅3(H2O)]n ⋅m(C12H22O11)
where: n is the degree of iron polymerization and m is the number of sucrose
molecules associated with the iron (III)-hydroxide.
Each mL contains 20 mg elemental iron as iron sucrose in water for injection.
8.0 Pharmaceutical particulars
1.Incompatibilities
Feronia-IV must not be mixed with other medicinal products except sterile 0.9% sodium chloride solution(NS) for dilution.
The diluted solution must appear as brown and clear.
Each ampoule of Feronia-IV is intended for single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
There is the potential for precipitation and/or interaction if mixed with other solutions or medicinal products. The compatibility with containers other than glass, polyethylene and PVC is not known.
2. Shelf-life
Refer on the pack.
3.Packaging information
Feronia-IV 50: A blister pack of 2.5 ml ampoule. Feronia-IV 100: A blister pack of 5 ml ampoule.
4.Storage and handing instructions
Ampoules or vials should be visually inspected for sediment and damage before use. Use only those containing a sediment free and homogenous solution.
Store in a cool & dry place. Protect from light.
Keep out of reach of children.
Store at controlled room temperature (15°C to 25°C). Do not refrigerate.
9.0 Patient Counselling Information
Prior History of Reactions to Parenteral Iron Products
Question the patients regarding any prior history of reactions to parenteral iron products.
Serious Hypersensitivity Reactions
Advise patients to report any symptoms of hypersensitivity that may develop during and following Feronia-IV administration, such as rash, itching, dizziness, light headedness, swelling, and breathing problems.
About leaflet
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
-Keep this leaflet. You may need to read it again.
-If you have any further questions, ask your doctor.
-If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1.What Feronia IV 50 & 100 Injection is and what it is used for
2.What you need to know before Feronia IV 50 & 100 Injection is given to you
3.How Feronia IV 50 & 100 Injection is given
4.Possible side effects
5.How to store Feronia IV 50 & 100 Injection?
6.Contents of the pack and other information
1. What Feronia IV 50 and 100 Injection is and what it is used for
Feronia IV 50 & 100 Injection is a medicine that contains iron. Medicines that contain iron are used when you do not have enough iron in your body. This is called “iron deficiency”. Feronia IV 50 and 100 Injection is given when:
-You cannot take iron by mouth
- such as when iron tablets make you feel ill.
-You have taken iron by mouth - and it has not worked.
2. What you need to know before Feronia IV 50 and 100 Injection is given to you
You must not receive Feronia IV 50 and 100 Injection if:
-You are allergic (hypersensitive) to the product or any of the other ingredients of this medicine (listed in section 6).
-You have experienced serious allergic (hypersensitive) reactions to other injectable iron preparations.
-You have anemia which is not caused by a shortage of iron.
-You have too much iron in your body or a problem in the way your body uses iron.
You must not be given Feronia IV 50 & 100 Injection if any of the above apply to you. If you are not sure, talk to your doctor before having Feronia IV 50 & 100 Injection.
Warnings and precautions
Talk to your doctor or nurse before receiving Feronia IV 50 & 100 Injection if:
-You have a history of medicine allergy.
-You have systemic lupus erythematosus.
-You have rheumatoid arthritis.
-You have severe asthma, eczema or other allergies.
-You have any infections.
-You have liver problems.
If you are not sure if any of the above apply to you, talk to your doctor before you are given Feronia IV 50 and 100 Injection.
Other medicines and Feronia IV 50 and 100 Injection
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines obtained without a prescription, including herbal medicines. This is because Feronia IV 50 & 100 Injection can affect the way some other medicines work. Also some other medicines can affect the way Feronia IV 50 and 100 Injection works.
In particular, tell your doctor if you are taking:
-Medicines that contain iron which you take by mouth. These may not work if they are taken at the same time that Feronia IV 50 & 100 Injection is given to you.
Pregnancy and breast-feeding
Feronia IV 50 & 100 Injection has not been tested in women who are in the first three months of their pregnancy. It is important to tell your doctor if you are pregnant, think you may be pregnant, or are planning to have a baby. If you become pregnant during treatment, you must ask your doctor for advice. Your doctor will decide whether or not you should be given this medicine.
If you are breast-feeding, ask your doctor for advice before you are given Feronia IV 50 & 100 Injection.
Ask your doctor or pharmacist for advice before taking any medicine, if you are pregnant or breast-feeding.
Driving and using machines
You may feel dizzy, confused or light-headed after being given Feronia IV 50 & 100 Injection. If this happens, do not drive or use any tool or machines. Ask your doctor if you are not sure.
Feronia IV 50 & 100 Injection contains Sodium
Feronia IV 50 & 100 Injection contains up to 7 mg sodium (main component of cooking/table salt) per mL. This is equivalent to 0.4% of the recommended maximum daily dietary intake of sodium for an adult.
3. How Feronia IV 50 and 100 Injection is given
Your doctor will decide how much Feronia IV 50 & 100 Injection to give you. He or she will also decide how often you need it and for how long. Your doctor will do a blood test to help work out the dose.
Your doctor or nurse will administer Feronia IV 50 and 100 Injection in one of the following ways:
-Slow injection into your vein
– 1 to 3 times per week.
-As an infusion (drip) into your vein
– 1 to 3 times per week. During dialysis
– it will be put into the venous line of the dialysis machine.
-Feronia IV 50 & 100 Injection will be administered in a structure where immunoallergic events can receive appropriate and prompt treatment.
You will be observed for at least 30 minutes by your doctor or nurse after each administration.
Feronia IV 50 and 100 Injection is a brown liquid and so the injection or infusion will look brown.
Use in children
Feronia IV 50 & 100 Injection is not recommended for use in children below 1 month of age.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions (uncommon)
If you have an allergic reaction, tell your doctor or nurse straight away. The signs may include:
- Low blood pressure (feeling dizzy, light-headed or faint).
- Swelling of your face.
- Difficulty breathing.
- Chest pain which can be a sign of a potentially serious allergic reaction called Kounis syndrome.
In some patients these allergic reactions (rare) may become severe or life-threatening (known as anaphylactoid/anaphylactic reactions). Tell your doctor or nurse straight away if you think you are having an allergic reaction.
Other side effects include:
Common (may affect up to 1 in 10 people)
- Changes in your taste such as a metallic taste. This does not usually last very long.
- Low blood pressure or high blood pressure.
- Feeling sick (nausea).
- Reactions around the site of injection/ infusion such as pain, irritation, itching, haematoma or discolouration following the leakage of the injection into the skin.
Uncommon (may affect up to 1 in 100 people)
- Headache or feeling dizzy.
- Stomach pain or diarrhea.
- Being sick (vomiting).
- Wheezing, difficulty in breathing.
- Itching, rash.
- Muscle spasms, cramps or pain.
- Tingling or “pins and needles”.
- Reduced sensation of touch.
- Vein inflammation.
- Flushing burning sensation.
- Constipation.
- Joint pain.
- Pain in limbs.
- Back pain.
- Chills.
- Weakness, tiredness.
- Swelling of hands and feet.
- Pain.
- Increased levels of liver enzymes (ALT, AST, GGT) in the blood.
- Increased serum ferritin levels.
Rare (may affect up to 1 in 1,000 people)
- Fainting.
- Sleepiness or drowsiness.
- Pounding heart beat (palpitations).
- Changes to the color of your urine.
- Chest pain.
- Increased sweating.
- Fever.
- Increased lactate dehydrogenase in the blood.
Other side effects with unknown frequency include: feeling less alert, feeling confused; loss of consciousness; anxiety; trembling or shaking; swelling of your face, mouth, tongue or throat which may cause difficulty in breathing; low pulse rate; fast pulse rate; circulatory collapse; vein inflammation causing the formation of a blood clot; acute narrowing of the airways; itching, hives, rash or skin redness; cold sweat; general feeling of illness; pale skin; sudden life-threatening allergic reactions. Flu-like illness may occur a few hours to several days after injection and is typically characterized by symptoms such as high temperature, and aches and pains in muscles and joints.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Feronia IV 50 and 100 Injection?
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the label after EXP. Do not store above 25oC. Do not freeze. Keep the ampoules or vials in the outer carton.
Once the Feronia IV 50 & 100 Injection ampoules have been opened, they should be used immediately. After dilution with sodium chloride solution, the diluted solution should be used immediately. Feronia IV 50 & 100 Injection will normally be stored for you by your doctor or the hospital.
6. Contents of the pack and other information
What Feronia IV 50 & 100 Injection contains
- The active substance is iron (as iron sucrose). Each millilitre (ml) contains 20 mg iron.
What Feronia IV 50 and 100 Injection looks like and contents of the pack
Feronia IV 50 and 100 Injection comes in following pack-sizes:
5 Blister packs of 5 mL Glass ampoules each. Each ampoule of 5 mL corresponds to 100 mg of iron.
Marketing Authorization Holder and Manufacturer
Zuventus Healthcare Limited
Zuventus House Plot No. Y2, CTS No: 358/A2,
near Nahur Railway Station, off Raycon IT Park Road,
Nahur West, Mumbai, Maharashtra 400078
This leaflet was last revised in 06/2023.
10.0 Date of revision
17th Sept. 2024


