Feronia Max Suspension
Therapy Area
Vitamins/Minerals Supplements
1.0 Generic name
Ferrous ascorbate, Folic acid & Methylcobalamin Suspension
2.0 Qualitative and quantitative composition
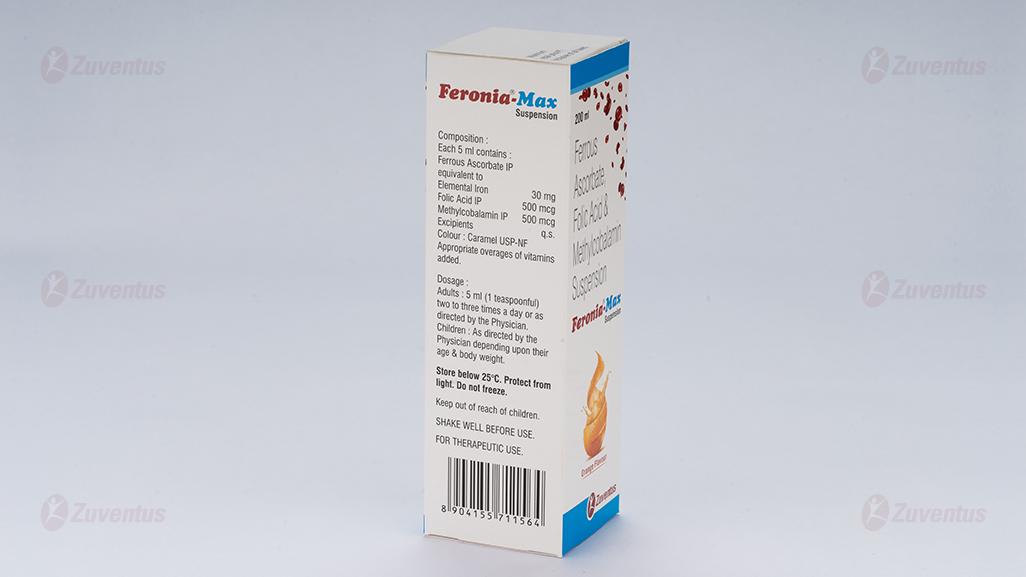
Each 5 ml contains:
Ferrous Ascorbate equivalent to Elemental Iron…… 30 mg
Folic acid IP……500 mcg
Methylcobalamin IP…….500 mcg
3.0 Dosage form and strength
200 ml bottle Suspension
[30 mg Ferrous ascorbate + 500 mcg Folic acid + 500 mcg Methylcobalamin] per 5 ml
4.0 Clinical particulars
4.1 Therapeutic indication
For the treatment of iron deficiency anaemia.
4.2 Posology and method of administration
1-2 teaspoonful daily (5-10 mL) or as directed by the physician.
Method of administration: Oral
4.3 Contraindications
- Patients hypersensitive to ferrous ascorbate, folic acid, methylcobalamin or to any other component of this formulation.
- Paroxysmal nocturnal haemoglobinuria, haemosiderosis, haemochromatosis.
- Repeated blood transfusions. Must not be used in anaemias other than those due to iron deficiency.
4.4 Special warnings and precautions for use
General
Do not exceed the recommended dose. The type of anaemia and the underlying cause or causes should be determined before starting therapy with this medication. Since the anaemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
- In general, people with a history of kidney disease, intestinal disease, peptic ulcer disease, enteritis, colitis, pancreatitis, hepatitis, who consume excessive alcohol, who plan to become pregnant, or who are over 55 years of age and have a family history of heart disease should consult a doctor and pharmacist before taking iron.
- Individuals with blood disorders who require frequent blood transfusions are also at risk of iron overload and should not take iron supplements without direction by a qualified healthcare provider. Long-term use of high doses of iron can cause haemosiderosis that clinically resembles haemochromatosis. Iron overload is associated with several genetic diseases, including haemochromatosis. Accumulation of excess iron is being investigated as a potential contributor to neurodegenerative diseases such as Alzheimer's and Parkinson's disease.
- Vitamin B12 deficiency that is allowed to progress for longer than 3 months may produce permanent degenerative lesions of the spinal cord.
- Folic acid in doses above 0.1 mg daily may obscure pernicious anaemia in that haematologic remission can occur while neurological manifestations remain progressive. This may result in severe nervous system damage before the correct diagnosis is made. Neurological manifestations will not be prevented with Folic acid, and if not treated with Vitamin B12, irreversible damage will result. Adequate doses of Vitamin B12 may prevent, halt, or improve the neurological changes caused by pernicious anaemia.
- Administration of Folic acid alone is improper therapy for pernicious anaemia and other megaloblastic anaemias in which Vitamin B12 is deficient. Doses of Cyanocobalamin exceeding 10 mcg daily may produce a hematologic response in patients with folate deficiency. Indiscriminate administration may mask the true diagnosis.
- Caution should be used in patients undergoing angioplasty since an intravenous loading dose of Folic acid, Vitamin B6, and Vitamin B12, followed by oral administration taken daily after coronary stenting, might actually increase restenosis rates. Due to the potential for harm, this combination of vitamins should not be recommended for patients receiving coronary stents.
- For pernicious anaemia, an adequate dose must be used and the blood picture must be examined regularly at least every three months for 18 months until stabilised, and then annually.
- Indiscriminate administration of this medicine may mask precise diagnosis.
Drug interactions
- The administration of the following with Iron salts results in decreased iron effectiveness : Aluminium Hydroxide, Aluminium Phosphate, Calcium, Aluminium Carbonate (basic), Chloramphenicol, Dihydroxyaluminium Aminoacetate, Dihydroxyaluminium Sodium Carbonate, Magaldrate, Magnesium Carbonate, Magnesium Hydroxide, Magnesium Oxide, Magnesium Trisilicate, Methacycline, Minocycline, Oxytetracycline, Rolitetracycline, Sodium Bicarbonate.
- The administration of the following with iron salts results in decreased effectiveness of the following molecules : Cefdinir, Cinoxacin, Ciprofloxacin, Enoxacin, Gatifloxacin, Gemifloxacin, Grepafloxacin, Levofloxacin, Lomefloxacin, Moxifloxacin, Norfloxacin, Ofloxacin, Penicillamine, Sparfloxacin, Temafloxacin, Trovafloxacin Mesylate, Levothyroxine.
- The administration of the following with iron salts results in decreased effectiveness of the molecule as well as Iron : Zinc (with decreased gastrointestinal absorption), Acetohydroxamic acid, Trientine (with decreased gastrointestinal absorption).
- The administration of the following with iron salts results in reduced iron absorption/bioavailability : Esomeprazole, Desferrioxamine, Gossypol, Lansoprazole, Omeprazole, Pantoprazole, Rabeprazole, Soybean, Soy Protein, Vanadium, Cholestyramine, Colestipol, Pancrelipase.
- The administration of the following molecules with iron decreases the absorption of the molecules : Ciprofloxacin, Ofloxacin, Levofloxacin, Tiludronate, Risedronate, Alendronate, Etidronate, Ibandronate, Levodopa, Methyldopa, Levothyroxine, Mycophenolic acid, Minocycline, Doxycycline, Tetracycline, Demeclocycline.
- Allopurinol may increase iron storage in the liver and should not be used in combination with iron supplements.
- Aminosalicylic acid may cause a malabsorption syndrome (weight loss, iron and vitamin depletion, steatorrhoea).
- Aspirin and NSAIDs can cause mucosal damage and bleeding throughout the gastrointestinal tract. Chronic blood loss associated with the long-term use of these agents may contribute to iron deficiency anaemia. Since iron supplements may also irritate the gastrointestinal tract, patients should not use them concurrently with NSAIDs unless recommended by a Physician.
- Chloramphenicol can reduce the response to iron therapy in iron deficiency anaemia.
- Iron supplements and dimercaprol may combine in the body to form a harmful chemical.
- Adequate dietary iron intake is recommended when taking H2 blockers like Cimetidine, Ranitidine, Famotidine or Nizatidine. Iron supplements are not usually required unless they are being used for another indication.
- The administration of the following with Folic acids results in decreased effectiveness of the molecule : Fosphenytoin, Phenytoin, Pyrimethamine.
- The administration of the following with folate results in reduced folate absorption: Aminosalicylic acid, Antacids, Antibiotics, Aspirin, Carbamazepine, Cholestyramine, Colestipol, Cycloserine, Cimetidine, Famotidine, Nizatidine, Ranitidine, Pancrelipase, Sulfasalazine, Triamterene.
- The administration of the following with folate results in reduced serum folate levels: Oestrogens conjugated, Oral contraceptives pills, Methotrexate, Methylprednisolone Sodium Succinate (noted in people with multiple sclerosis), Intravenous pentamidine, Phenobarbital and Primidone, Pyrimethamine.
- Excessive use of Alcohol increases the requirement for Folic acid.
- Chloramphenicol may antagonize some effects of Folic acid on the blood (haematopoietic system). Limited data suggests that diuretics may increase excretion of Folic acid.
- Reduced Vitamin B12 and, to a lesser extent, folate levels occur in some people with diabetes and can contribute to hyperhomocysteinaemia, which adds to the already increased risk of cardiovascular disease. The reduced folate levels seen in diabetics have been linked to Metformin use in some cases, possibly as a result of reduced Folic acid absorption. Symptomatic folate deficiency is unlikely to occur with Metformin, but people with diabetes may need Folic acid supplements to reduce hyperhomocysteinaemia.
- Chronic cigarette smoking is associated with diminished folate status.
- Folate-dependent enzymes have been inhibited in laboratory experiments by Ibuprofen, Naproxen, Indomethacin and Sulindac.
- One study found that administration of Folic acid to pregnant women might not interfere with the protective effect of the sulphadoxine/pyrimethamine combination when used for intermittent preventative treatment of malaria.
- There is a general belief that Folic/folinic acid supplements do not interfere with the therapeutic effects of trimethoprim. However, this view has been challenged, and failure of trimethoprim therapy has occurred rarely when folinic acid is given concurrently.
- Absorption may be reduced by Para-aminosalicylic acid, Colchinine, Biguanides, Neomycin, Cholestyramine, Potassium Chloride, Methyldopa, and Cimetidine.
- Patients treated with Chloramphenicol may respond poorly to this medicine.
- Serum levels of this medicine may be lowered by oral contraceptives. These interactions are unlikely to have clinical significance.
- Anti-metabolities and most antibiotics invalidate Vitamins B12 assays by microbiological techniques.
Special populations
Pregnancy
Nutritional supplement doses of vitamins and minerals are generally considered safe during pregnancy.
Lactation
For the prevention and treatment of iron deficiency and to supply a maintenance dosage of folic acid.
4.7 Effects on ability to drive and use machines
There is no clinical study of Feronia Max Suspension in drive and use machines.
4.8 Undesirable effects
Adverse reactions with iron therapy are usually transient. Gastrointestinal upset, including nausea, vomiting, constipation, diarrhoea and dark stools, has been reported. Gastrointestinal side effects are relatively common and corrective bowel regimens such as increasing dietary fibre or over-the-counter medications might be recommended to balance these side effects.
Folate appears to be well-tolerated in recommended doses. Stomatitis, alopecia, myelosuppression and zinc depletion have been reported.
Erythema, urticaria, skin flushing, rash, itching, bloating, flatulence, cramps, bitter taste, and diarrhoea have been reported. The colour of the urine may become more intense. Irritability, excitability, general malaise, altered sleep patterns, vivid dreaming, overactivity, confusion, impaired judgment, increased seizure frequency, and psychotic behaviour have been reported. Very high doses can cause significant central nervous system side effects. Supplemental Folic acid might increase seizures in people with seizure disorders, particularly in very high doses.
Anaphylaxis and bronchospasm have also been reported.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website : https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
The clinical course of acute iron overdosage can be variable. Initial symptoms may include abdominal pain, nausea, vomiting, diarrhoea, tarry stools, melena, haematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycaemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock and coma.
Acute overdosage or iron accumulation symptoms may include arthritis, signs of gonadal failure (amenorrhoea, early menopause, loss of libido, impotence), and shortness of breath/dyspnoea. High doses may cause vomiting and diarrhoea, followed by cardiovascular or metabolic toxicity and death. It is unclear whether high levels are associated with cancer, coronary heart disease or myocardial infarction (MI or heart attack).
The stomach should be emptied by aspiration and gastric lavage if the patient reports immediately after overdosage.
Treatment should be symptomatic and supportive.
5.0 Pharmacological properties
5.1 Mechanism of action / pharmacodynamic properties
Iron salts
Administration of iron in the form of Ferrous Ascorbate delivers maximum amount of Ferrous iron to the duodenal brush border and produces minimum GI adverse effects. Mechanism of action of Ferrous Ascorbate :
- With Ferrous Ascorbate iron absorption is facilitated by formation of soluble iron Ascorbate complexes and by inhibition of formation of insoluble iron complexes.
- It inhibits the conversion of Ferrous to Ferric iron which increases iron absorption.
- It also inhibits the effect of phytates, phosphates and oxalates on iron absorption.
- Iron is mobilized from the core ferritin to the sites of erythropoiesis and at the same time conversion of ferritin to hemosiderin (which cannot be utilized) is inhibited.
- In this way Ferrous Ascorbate allows rapid correction of haemoglobin.
- Inhibition of conversion of Ferrous to ferric iron reduces the amount of free radicals generated, thus, minimizing the GI adverse effects.
Folic acid
Folic acid is required to maintain normal erythropoiesis and nucleoprotein synthesis. Folic acid is reduced by enzymes folate reductase and Dihydrofolate reductase to form Dihydrofolic acid and Tetrahydrofolic acid respectively. Tetrahydrofolic acid acts as a coenzyme which mediates a number of one carbon transfer reactions by carrying a methyl group as an adduct. It involves a number of reactions such as :
- Conversion of homocysteine to methionine.
- Synthesis of thymidylate, which is an essential constituent of DNA, from Methylene-Tetrahydrofolic acid.
- Conversion of serine to glycine by Tetrahydrofolic acid and formation of Methylene-Tetrahydrofolic acid.
- To introduce carbon units at position 2 and 8 during de novo purine synthesis requires Formyl-Tetrahydrofolic acid and Methenyl Tetrahydrofolic acid.
- Generation and utilization of "formate pool".
- For mediating formino group transfer in histidine metabolism.
Methylcobalamin
Vitamin B12 is found only in foods of animal origin. Vitamin B12 is essential for cellular DNA synthesis and hence contributes to functions of various tissues of the body, formation of myelin sheath, more so the rapidly dividing and proliferating cellular systems such as blood and gastric epithelium. The absorbed inert form of cobalamin is converted into two important active forms. One is Methylcobalamin-involved in maturation of red blood corpuscles. The second active form is Adenosylcobalamin involved in healthy myelination and neuronal integrity.
5.2 Pharmacokinetic properties
Ferrous Ascorbate
The bioavailability of reference dose Ferrous Ascorbate has been reported in the range of 26.4% to 50.4%. Multiple studies have proven bioavailability of Ferrous Ascorbate to be better than other iron salts. Ferrous Ascorbate salt is more resistant to oxidation at alkaline pH and thus, delivers maximum amount of Ferrous iron to the duodenal brush border leading to increased iron absorption. Ferrous form is absorbed thrice as much as ferric form of iron. Following absorption, iron is transported in a transferrin bound form to bone marrow. Excretion is minimal.
Folic acid
Folic acid is absorbed rapidly from the small intestine, primarily from the proximal portion. It appears in the plasma approximately 15 to 30 minutes after an oral dose; peak levels are generally reached within 1 hour. Folic acid is metabolized in the liver to 7,8-dihydroFolic Acid and eventually to 5,6,7,8-tetrahydrofolic Acid with the aid of reduced diphosphopyridine nucleotide (DPNH) and folate reductases. Tetrahydrofolic Acid derivatives are distributed to all body tissues but are stored primarily in the liver. Folic acid has a half-life of 2 hours and is excreted mainly via urine.
Methylcobalamin
The absorption of cobalamins from the gut is dependent upon the glycoprotein intrinsic factor. Cobalamins are transported rapidly into the blood bound to protein, known as Transcobalamins. Cobalamins are stored in the liver and excreted in the bile. They are known to cross the placenta.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
As per the toxicity reports, designed to determine the acute oral toxicity of Ferrous Ascorbate Complex to Sprague Dawley rats. No signs of intoxication were observed in animals treated at the dose level of 2000 mg/kg of the test substance. All animals survived through the study period of 14 days. Animals from control and 2000 mg/kg dose group exhibited normal body weight gain on day 7 and day 14. Gross pathological examination did not reveal any abnormalities attributable to the treatment.
7.0 Description
Feronia Max Suspension is the combination of Ferrous Ascorbate, Folic acid and Methylcobalamin.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
24 Months
8.3 Packaging information
1 x 200 ml
8.4 Storage and handing instructions
Store below 30°C. Protect from light. Do not freeze.
KEEP AWAY FROM THE REACH OF CHILDREN. Shake well before use.
9.0 Patient counselling information
- Ask the patient to report any adverse events.
- Not to exceed the stated recommended daily dose.
12.0 Date of issue
1st July 2024