Fevridol 1gm IV
Therapy Area
Antipyretic
1.0 Generic Name
Paracetamol Infusion IP 1.0% w/v
2.0 Qualitative and quantitative composition
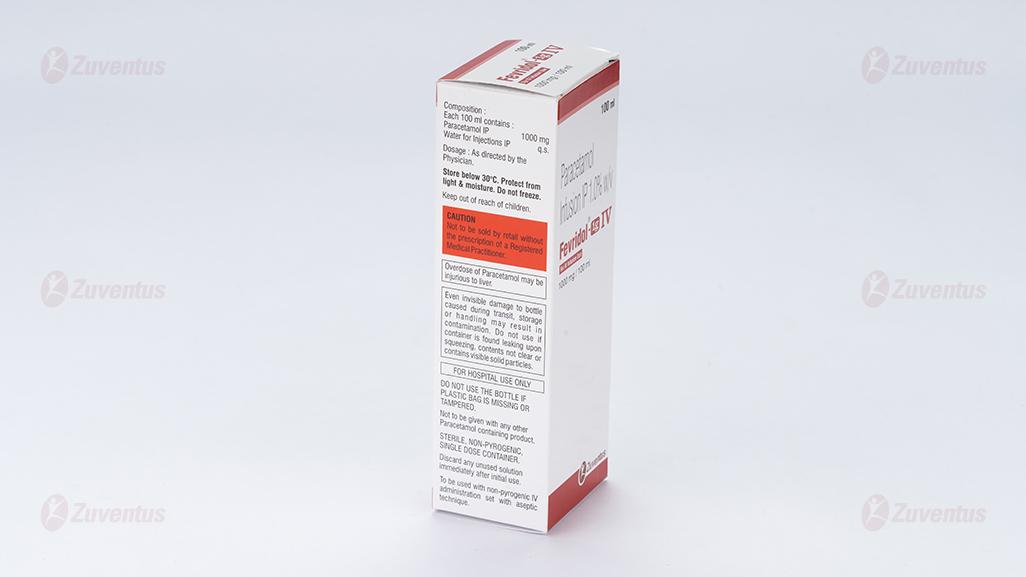
Each 100 ml contains:
Paracetamol IP 1000 mg
Water for Injections IP q.s.
3.0 Dosage form and strength
Solution for infusion.
1000mg/100ml
4.0 Clinical particulars
4.1. Therapeutic indications
For short term treatment of moderate pain, especially following surgery and for the short term treatment of fever, when administration by intravenous route is clinically justified.
4.2. Posology and method of administration
Dosing based on patient weight.
| Patient weight | Dose per administration | Volume per administration | Maximum Volume of Paracetamol (10 mg/ml) per administration based on upper weight of group (ml)*** | Maximum daily dose** |
| ≤ 10 kg* | 7.5 mg/kg | 0.75 ml/kg | 7.5 ml | 30 mg/kg |
| > 10 kg to ≤ 33 kg | 15 mg/kg | 1.5 ml/kg | 49.5 ml | 60 mg/kg not exceeding 2 g |
| > 33 kg to ≤ 50 kg | 15 mg/kg | 1.5 ml/kg | 75 ml | 60 mg/kg not exceeding 3g |
| > 50 kg with additional risk factors for hepatotoxicity | 1 g | 100 ml | 100 ml | 3 g |
| > 50 kg and no additional risk factors for hepatotoxicity | 1 g | 100 ml | 100 ml | 4 g |
* Preterm new-born infants:
No safety and efficacy data are available for premature newborn infants
** Maximum daily dose:
The maximum daily dose as presented in the table above is for patients that are not receiving other paracetamol containing products and should be adjusted accordingly taking such products into account.
*** Patients weighing less will require smaller volumes.
The minimum interval between each administration must be at least 4 hours.
The minimum interval between each administration in patients with severe renal insufficiency must be at least 6 hours.
No more than 4 doses to be given in 24 hours.
Severe renal insufficiency:
It is recommended, when giving paracetamol to patients with severe renal impairment (creatinine clearance ≤ 30 ml/min), to reduce the dose and increase the minimum interval between each administration to 6 hours.
Adults with hepatocellular insufficiency, chronic alcoholism, chronic malnutrition (low reserves of hepatic glutathione), dehydration:
The maximum daily dose must not exceed 3000 mg.
Method of administration
Intravenous use.
The paracetamol solution is administered as a 15-minute intravenous infusion.
Take care when prescribing and administering Paracetamol to avoid dosing errors due to confusion between milligram (mg) and millilitre (ml), which could result in accidental overdose and death. Take care to ensure the proper dose is communicated and dispensed. When writing prescriptions, include both the total dose in mg and the total dose in volume. Take care to ensure the dose is measured and administered accurately.
Patients weighing ≤ 10 kg:
● The volume to be administered should be withdrawn and diluted in a sodium chloride (0.9%) solution or glucose (5%) solution up to one tenth (one volume Paracetamol into nine volumes diluent) and administered over 15 minutes.
● A 5 or 10 ml syringe should be used to measure the dose as appropriate for the weight of the child and the desired volume. However, this should never exceed 7.5 ml per dose
● The user should be referred to the product information for dosing guidelines. Paracetamol can be diluted in (0.9%) sodium chloride solution or (5%) glucose solution or a combination of both solutions up to one tenth (one volume Paracetamol into nine volumes diluent). In this case, use the diluted solution within the hour following its preparation (infusion time included).
For single use only. Any unused solution should be discarded.
Before administration, the product should be visually inspected for any particulate matter and discolouration. Only to be used if solution is clear, and the container and its closure are undamaged.
As for all solutions for infusion presented in containers with air space inside, it should be remembered that close monitoring is needed notably at the end of the infusion, regardless of administration route. This monitoring at the end of the infusion applies particularly for central route infusions, in order to avoid air embolism.
4.3. Contraindications
- Hypersensitivity to paracetamol, or to any of the excipients.
- Cases of severe hepatocellular insufficiency.
4.4.Special warnings and precautions for use
RISK OF MEDICATION ERRORS
Take care to avoid dosing errors due to confusion between milligram (mg) and milliliter (ml), which could result in accidental overdose and death
rolonged or frequent use is discouraged. It is recommended that a suitable analgesic oral treatment will be used as soon as this route of administration is possible.
In order to avoid the risk of overdose, check that other medicines administered do not contain either paracetamol. The dose may require adjustment.
Doses higher than those recommended entail the risk of very serious liver damage. Clinical signs and symptoms of liver damage (including fulminant hepatitis, hepatic failure, cholestatic hepatitis, cytolytic hepatitis) are usually first seen after two days of drug administration with a peak seen, usually after 4 – 6 days. Treatment with antidote should be given as soon as possible.
Paracetamol should be used with caution in cases of:
● hepatocellular insufficiency
● severe renal insufficiency (creatinine clearance ≤ 30 ml/min)
● chronic alcoholism
● chronic malnutrition (low reserves of hepatic glutathione)
● dehydration
● patients suffering from a genetically caused G-6-PD deficiency (favism), the occurrence of a haemolytic anaemia is possible due to the reduced allocation of glutathione following the administration of paracetamol.
Caution is advised if paracetamol is administered concomitantly with flucloxacillin due to increased risk of high anion gap metabolic acidosis (HAGMA), particularly in patients with severe renal impairment, sepsis, malnutrition and other sources of glutathione deficiency (e.g. chronic alcoholism), as well as those using maximum daily doses of paracetamol. Close monitoring, including measurement of urinary 5-oxoproline, is recommended.
4.5. Interaction with other medicinal products and other forms of interaction
- Probenecid causes an almost two-fold reduction in clearance of paracetamol by inhibiting its conjugation with glucuronic acid. A reduction in the paracetamol dose should be considered if it is to be used concomitantly with probenecid.
- Salicylamide may prolong the elimination half-life of paracetamol.
- Caution should be taken with the concomitant intake of enzyme-inducing substances.
- Concomitant use of paracetamol (4000 mg per day for at least 4 days) with oral anticoagulants may lead to slight variations of INR values. In this case, increased monitoring of INR values should be conducted during the period of concomitant use as well as for 1 week after paracetamol treatment has been discontinued.
- Caution should be taken when paracetamol is used concomitantly with flucloxacillin as concurrent intake has been associated with high anion gap metabolic acidosis, especially in patients with risks factors.
4.6. Fertility, pregnancy and lactation
Pregnancy:
A large amount of data on pregnant women indicate neither malformative, nor feto/neonatal toxicity. Epidemiological studies on neurodevelopment in children exposed to paracetamol in utero show inconclusive results. If clinically needed, paracetamol can be used during pregnancy however it should be used at the lowest effective dose for the shortest possible time and at the lowest possible frequency.
Lactation:
After oral administration, paracetamol is excreted into breast milk in small quantities. No undesirable effects on nursing infants have been reported. Consequently, Paracetamol may be used in breast-feeding women.
4.7.Effects on ability to drive and use machines
Not relevant.
4.8.Undesirable effects
As with all paracetamol products, adverse drug reactions are rare (≥ 1/10 000 to <1/1 000) or very rare (<1/10 000). They are described below:
| System Organ Class | Common | Rare (≥ 1/10 000 to <1/1 000) | Very rare (<1/10 000) | Not known (cannot be estimated from the available data) |
| Blood and lymphatic system disorders | — | Thrombocytopenia, Leucopenia, Neutropenia | — | |
| Immune system disorders | — | Hypersensitivity reaction/td> | — | |
| Cardiac disorders | — | — | Tachycardia | |
| Vascular disorders | Hypotension | — | Flushing | |
| Hepatobiliary disorders | Increased levels of hepatic transaminases | — | — | |
| Skin and subcutaneous tissue disorders | — | serious skin reactions; | Pruritus, Erythema | |
| General disorders and administration site conditions | Malaise | — | — |
(1) Very rare cases of hypersensitivity reactions ranging from simple skin rash or urticaria to anaphylactic shock have been reported and require discontinuation of treatment.
(2) Isolated cases
(3) Very rare cases of serious skin reactions have been reported.
Frequent adverse reactions at injection site have been reported during clinical trials (pain and burning sensation).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9.Overdose
Symptoms
There is a risk of liver injury (including fulminant hepatitis, hepatic failure, cholestatic hepatitis, cytolytic hepatitis), particularly in elderly subjects, in young children, in patients with liver disease, in cases of chronic alcoholism, in patients with chronic malnutrition and in patients receiving enzyme inducers. Overdosing may be fatal in these cases.
Symptoms generally appear within the first 24 hours and comprise: nausea, vomiting, anorexia, pallor and abdominal pain. Immediate emergency measures are necessary in case of paracetamol overdose, even when no symptoms are present.
Overdose, 7.5 g or more of paracetamol in a single administration in adults or 140 mg/kg of body weight in a single administration in children, causes hepatic cytolysis likely to induce complete and irreversible necrosis, resulting in hepatocellular insufficiency, metabolic acidosis and encephalopathy which may lead to coma and death. Simultaneously, increased levels of hepatic transaminases (AST, ALT), lactate dehydrogenase and bilirubin are observed together with decreased prothrombin levels that may appear 12 to 48 hours after administration. Clinical symptoms of liver damage are usually evident initially after two days, and reach a maximum after 4 to 6 days.
Treatment
Immediate hospitalisation.
Before beginning treatment, take a blood sample for plasma paracetamol assay, as soon as possible after the overdose. The treatment includes administration of the antidote, N-acetylcysteine (NAC) by the intravenous or oral route, if possible before the 10th hour. NAC can, however, give some degree of protection even after 10 hours, but in these cases prolonged treatment is given.
Symptomatic treatment.
Hepatic tests must be carried out at the beginning of treatment and repeated every 24 hours. In most cases hepatic transaminases restitution to normal in one to two weeks with full return of normal liver function. In very severe cases, however, liver transplantation may be necessary.
5.0 Pharmacological properties
Pharmacotherapeutic group:
Analgesics; Other analgesics and antipyretics; Anilides
ATC Code: N02BE01
5.1 Mechanism of action
The precise mechanism of the analgesic and antipyretic properties of paracetamol has still to be established; it may involve central and peripheral actions.
5.2 Pharmacodynamic effects
Paracetamol provides onset of pain relief within 5 to 10 minutes after the start of administration. The peak analgesic effect is obtained in 1 hour and the duration of this effect is usually 4 to 6 hours.
Paracetamol reduces fever within 30 minutes after the start of administration with a duration of the antipyretic effect of at least 6 hours.
5.3 Pharmacokinetic properties
Adults
Absorption:
Paracetamol pharmacokinetics is linear up to 2 g after single administration and after repeated administration during 24 hours.
The bioavailability of paracetamol following infusion of 500 mg and 1 g of Paracetamol is similar to that observed following infusion of 1 g and 2 g propacetamol (containing 500mg and 1 g paracetamol respectively). The maximal plasma concentration (Cmax) of paracetamol observed at the end of 15-minutes intravenous infusion of 500 mg and 1 g of Paracetamol is about 15 μ g/ml and 30 μ g/ml respectively.
Distribution:
The volume of distribution of paracetamol is approximately 1 l/kg.
Paracetamol is not extensively bound to plasma proteins.
Following infusion of 1 g paracetamol, significant concentrations of paracetamol (about 1.5 µ g/ml) were observed in the cerebrospinal fluid at and after the 20th minute following infusion.
Biotransformation:
Paracetamol is metabolised mainly in the liver following two major hepatic pathways: glucuronic acid conjugation and sulphuric acid conjugation. The latter route is rapidly saturable at doses that exceed the therapeutic doses. A small fraction (less than 4 %) is metabolised by cytochrome P450 to a reactive intermediate (N-acetyl benzoquinone imine) which, under normal conditions of use, is rapidly detoxified by reduced glutathione and eliminated in the urine after conjugation with cysteine and mercapturic acid. However, during massive overdosing, the quantity of this toxic metabolite is increased.
Elimination:
The metabolites of paracetamol are mainly excreted in the urine. 90 % of the dose administered is excreted within 24 hours, mainly as glucuronide (60 – 80%) and sulphate (20 – 30 %) conjugates. Less than 5 % is eliminated unchanged. Plasma half-life is 2.7 hours and total body clearance is 18 l/h.
Newborn infants, infants and children:
The pharmacokinetic parameters of paracetamol observed in infants and children are similar to those observed in adults, except for the plasma half-life that is slightly shorter (1.5 to 2 h) than in adults. In newborn infants, the plasma half-life is longer than in infants i.e. around 3.5 hours. Newborn infants, infants and children up to 10 years excrete significantly less glucuronide and more sulphate conjugates than adults.
Table - Age related pharmacokinetic values
(standardised clearance, *CLstd/Foral (l× h-1× 70 kg-1)
| Age | Weight (kg) | CLstd/Foral (l× h-1× 70 kg-1) |
| 40 weeks post-conception | 3.3 | 5.9 |
| 3 months postnatal | 6 | 8.8 |
| 6 months postnatal | 7.5 | 11.1 |
| 1 year postnatal | 10 | 13.6 |
| 2 years postnatal | 12 | 15.6 |
| 5 years postnatal | 20 | 16.3 |
| 8 years postnatal | 25 | 16.3 |
*CLstd is the population estimate for CL
Special populations:
Renal insufficiency:
In cases of severe renal impairment (creatinine clearance 10–30 ml/min), the elimination of paracetamol is slightly delayed, the elimination half-life ranging from 2 to 5.3 hours. For the glucuronide and sulphate conjugates, the elimination rate is 3 times slower in subjects with severe renal impairment than in healthy subjects. Therefore, when giving paracetamol to patients with severe renal impairment (creatinine clearance ≤ 30 ml/min), the minimum interval between each administration should be increased to 6 hours.
Elderly subjects:
The pharmacokinetics and the metabolism of paracetamol are not modified in elderly subjects. No dose adjustment is required in this population.
6.0 Nonclinical properties
Non-clinical data reveal no special hazard for humans beyond the information included in other sections of the SmPC.
Studies on local tolerance of paracetamol in rats and rabbits showed good tolerability. Absence of delayed contact hypersensitivity has been tested in guinea pigs. Conventional studies using the currently accepted standards for the evaluation of toxicity to reproduction and development are not available.
7.0 Description
Acetaminophen is a non-salicylate antipyretic and non-opioid analgesic agent. Its chemical name is N-acetyl-p-aminophenol.
Acetaminophen has a molecular weight of 151.16.
Its structural formula is:

8.0 Pharmaceutical particulars
1. Incompatibilities
Paracetamol must not be mixed with other medicinal products except those mentioned in dosage administration section.
2. Shelf-life
Refer on the pack. After dilution: Chemical and physical in use stability (including infusion time) in the solutions listed in dosage administration section has been demonstrated for 48 hours at 23 ° C. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
3. Packaging information
A bottle of 100 ml.
4. Storage and handing instructions
Store below 30°C. Protect from light & moisture. Do not freeze.
Keep out of reach of children.
Before administration, the product should be visually inspected for any particulate matter and discoloration. The product is intended for single use only. The product should be used immediately after opening and any unused solution should be discarded.
About leaflet
Keep this leaflet. You may need to read it again.
If you have any further questions, please ask your child’s doctor, pharmacist or nurse.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What is Paracetamol and what it is used for
2. What do you know before you use Paracetamol
3. How to use Paracetamol
4. Possible side effects
5. How to store Paracetamol
6. Contents of the pack and other Information
1. What is Paracetamol and What It is Used for
This medicine is an analgesic (it relieves pain) and an antipyretic (it lowers fever). It is indicated for the short-term treatment of moderate pain, especially following surgery, and for the short-term treatment of fever.
The 100 ml container is restricted to adults, adolescents and children weighing more than 33 kg.
2. What Do You Know Before You Use Paracetamol
You should not be given Paracetamol:
If you are allergic to paracetamol or any of the other ingredients listed in the formulation or to propacetamol hydrochloride (prodrug of paracetamol).
If you are allergic to propacetamol (another analgesic and a precursor of paracetamol).
If you suffer from a severe liver disease.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before taking Paracetamol
- If you suffer from a liver or kidney disease, or from alcohol abuse.
- If you are taking other medicines containing paracetamol.
- In cases of nutrition problems (malnutrition) or dehydration. It is recommended that a suitable analgesic oral treatment be used as soon as this route of administration is possible.
Other medicines and Paracetamol
Tell your doctor or pharmacist if you are taking or have recently taken any other medicine. Do not give anything else containing paracetamol while giving this medicine. This medicine contains paracetamol and this must be taken into account if other medicines containing paracetamol or propacetamol are taken, in order not to exceed the recommended daily dose. Inform your doctor if you are taking other medicines containing paracetamol or propacetamol.
A dose reduction should be considered for concomitant treatment with probenecid.
Please inform your doctor or pharmacist if you are taking oral anticoagulants. More check-ups to look at the effect of the anticoagulant might be needed.
Pregnancy and Breastfeeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Inform your doctor if you are pregnant. Paracetamol may be used during pregnancy. However, in this case the doctor must evaluate if the treatment is advisable. Paracetamol may be used during breast-feeding.
Driving and using machines
The product does not affect the ability to drive or use machines
3. How to Use Paracetamol
Paracetamol will be administered to you by a healthcare professional.
The recommended dose is
Patients weighing more than 50 kg: Paracetamol 1 g per administration, i.e. one 100 mL vial, up to four times a day.
Patients weighing less than 50 kg and more than 33 kg: Paracetamol 15 mg/kg per administration (1.5 mL solution per kg) up to four times a day.
The minimum interval between each administration must be 4 hours in patients without hepatic or renal impairment. In patients with renal and/or hepatic impairment the minimum interval between doses must not be less than 6 hours.
For adults weighing from 33 to 50 kg the maximum daily dose from all sources of paracetamol must not exceed 60 mg/kg.
Neonates, infants and children weighing up to 33 kg (about 11 years old): Paracetamol 15 mg/kg per administration, i.e. 1.5 mL of solution per kg, up to four times a day.
The minimum interval between each administration must be 6 hours. The maximum daily dose must not exceed 60 mg/kg.
Term newborn infants, infants, toddlers and children weighing less than 10 kg (up to approximately 1-year-old): Reduce the dosage by half, i.e. 7.5 mg/kg paracetamol per administration, without exceeding four administrations per day.
Patients with hepatic impairment: In patients with chronic or active hepatic disease, especially those with hepatocelluar insufficiency, chronic alcoholism, chronic malnutrition (low reserves of hepatic glutathione) and dehydration the dose should not exceed 3 g/day.
Method of Administration
The paracetamol infusion is administered as a 15-minute intravenous infusion; it contains no antimicrobial agent and is for single use in one patient only. Paracetamol infusion can also be diluted in a 0.9% Sodium Chloride or 5% Glucose solution up to one tenth. In this case, use the diluted solution within the hour following its preparation (infusion time included).
If you take more Paracetamol then you should
If you or your child use more Paracetamol infusion than if you or your child should use, talk to a doctor at once if you or your child take too much of this medicine even if you or your child seem feel well. This is because too much paracetamol can cause delayed, serious liver damage. In overdose cases, symptoms generally appear within the first 24 hours and comprise: nausea, vomiting, anorexia, pallor, abdominal pain and risk of liver injury. Immediate medical advice should be sought in the event of overdosage, because of the risk of irreversible liver damage. Please inform your doctor if you notice any of these symptoms.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Occurrence > 1% (In adults)
Neurological : Dizziness, headache; Gastrointestinal : Vomiting, diarrhea, constipation, nausea, dyspepsia, enlarged abdomen, gastrointestinal disorder NOS; Hematological : Anemia, post-operative haemorrhage; Hepatobiliary : Gamma GT increase, SGPT increase; Skin and Appendage : Injection site pain, injection site reaction, post-operative site reaction, pruritus; Respiratory : Alveolitis, coughing; Endocrine/Metabolic : Hyperglycemia, hypokalaemia; General : Fatigue, chest pain
Occurrence > 1% (In children)
Skin and Appendage : Injection site pain, injection site reaction; Neurological : Hypotonia; Gastrointestinal : Nausea, vomiting; Body as a whole : Fever.
Rare (Occurrence 0.01 - 0.1%)
General : Malaise; Cardiovascular : Hypotension; Liver : Increased levels of hepatic transaminases; Platelet/Blood : Agranulocytosis, neutropenia; Skin and Appendage : Macular rash, injection site reaction
Very rare (Occurrence < 0.01%)
General : Hypersensitivity reaction; Renal/Genitourinary : Acute renal failure; Skin and Appendage : Maculo-papular rash, pemphigoid reaction, pustular rash
Reporting of side effects: If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
By reporting side effects you can also provide more information on the safety of this medicine.
5. How to Store Paracetamol
Store below 30°C. Protected from light & moisture. Do not freeze.
Keep out of reach of children.
Not to be sold by retail without the prescription of a Registered Medical Practitioner.
Even invisible damage to bottle caused during transit, storage or handling may result in contamination.
Do not use if container is found leaking upon squeezing, contents not clear or contains visible solid particles.
Do not use the bottle if plastic bag is missing or tampered.
Not to be given with any other Paracetamol containing product.
Discard any unused solution immediately after initial use.
6. Contents of the pack and other information
Each 100 ml contains :
Paracetamol IP 1000 mg
Water for Injections IP q.s.
9.0 Patient Counselling Information
- Paracetamol Injection helps in relieving moderate pain and fever for the short-term, especially following surgery. Paracetamol Injection has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of Paracetamol at doses that exceed the recommended maximum daily limits, and often involve more than one Paracetamol -containing product.
- Ask patient to inform doctor if also taking other medications containing paracetamol. Inform your doctor if you suffer from liver disease, severe kidney disease, or alcohol abuse.
- Inform your doctor if you experience symptoms such as feeling or being sick, weight loss, pale skin (pallor), or abdominal pain within the first 24 hours as it indicates an overdose.
- Your doctor may regularly monitor your kidney function, liver function and levels of blood components if you are taking this medicine for long-term treatment.
12.0 Date of revision
18th Sept. 2024