Fibricor Injection
Therapy Area
Cardiology
1.0 Name of the medicinal product
Ibutilide Fumarate Injection 0.1 mg/ml
2.0 Qualitative and quantitative composition
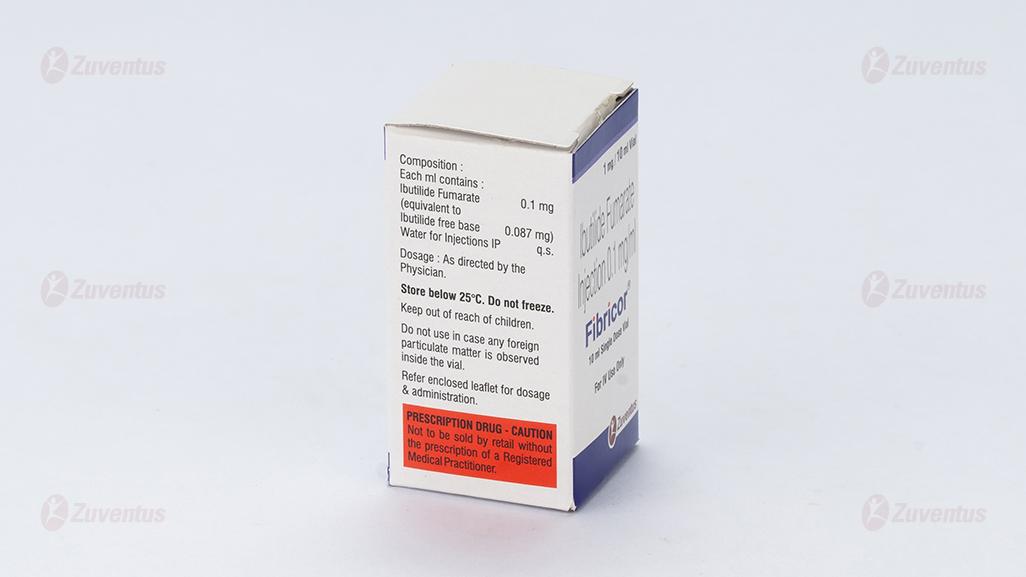
Each ml contains:
Ibutilide Fumarate……………………....0.1mg
(Equivalent to Ibutilide free base………0.087mg)
Water for Injections IP ……………. q.s.
3.0 Dosage form and strength
Intravenous infusion
0.1 mg/ml
4.0 Clinical Particulars
4.1 Therapeutic indication
For the rapid conversion of atrial fibrillation or atrial flutter of recent onset to sinus rhythm.
Patients with atrial arrhythmias of longer duration are less likely to respond to Ibutilide fumarate injection. The effectiveness of Ibutilide has not been determined in patients with arrhythmias of more than 90 days in duration.
LIFE-THREATENING ARRHYTHMIAS-APPROPRIATE TREATMENT ENVIRONMENT
Ibutilide fumarate injection can cause potentially fatal arrhythmias, particularly sustained polymorphic ventricular tachycardia, usually in association with QT prolongation (torsades de pointes), but sometimes without documented QT prolongation. In registration studies, these arrhythmias, which require cardioversion, occurred in 1.7% of treated patients during, or within a number of hours of, use of Ibutilide fumarate injection. These arrhythmias can be reversed if treated promptly (see WARNINGS, Proarrhythmia). It is essential that Ibutilide fumarate injection be administered in a setting of continuous ECG monitoring and by personnel trained in identification and treatment of acute ventricular arrhythmias, particularly polymorphic ventricular tachycardia. Patients with atrial fibrillation of more than 2 to 3 days duration must be adequately anticoagulated, generally for at least 2 weeks.
CHOICE OF PATIENTS
Patients with chronic atrial fibrillation have a strong tendency to revert after conversion to sinus rhythm and treatments to maintain sinus rhythm carry risks. Patients to be treated with Ibutilide fumarate injection, therefore, should be carefully selected such that the expected benefits of maintaining sinus rhythm outweigh the immediate risks of Ibutilide fumarate injection, and the risks of maintenance therapy, and are likely to offer an advantage compared with alternative management.
4.2 Posology and method of administration
The recommended dose of FIBRICOR Injection based on controlled trials is outlined in the Table below. Ibutilide Fumarate infusion should be stopped as soon as the presenting arrhythmia is terminated or in the event of sustained or non-sustained ventricular tachycardia, or marked prolongation of QT or QTc.
Recommended Dose of FIBRICOR Injection

In a trial comparing Ibutilide and sotalol, 2 mg butilide Fumarate administered as a single infusion to patients weighing more than 60 kg was also effective in terminating atrial fibrillation or atrial flutter.
In a post-cardiac surgery study, one or two intravenous infusions of 0.5 mg (0.005 mg/kg per dose for patients weighing less than 60 kg) was effective in terminating atrial fibrillation or atrial flutter.
Patients should be observed with continuous ECG monitoring for at least 4 hours following infusion or until QTc has returned to baseline. Longer monitoring is required if any arrhythmic activity is noted.
Skilled personnel and proper equipment, such as a cardioverter/defibrillator, and medication for treatment of sustained ventricular tachycardia, including polymorphic ventricular tachycardia, must be available during administration of FIBRICOR and subsequent monitoring of the patient.
Dilution
FIBRICOR Injection may be administered undiluted or diluted in 50 mL of diluent. FIBRICOR may be added to 0.9% Sodium Chloride Injection or 5% Dextrose Injection before infusion.

4.3 Contraindications
Ibutilide fumarate injection is contraindicated in patients who have previously demonstrated hypersensitivity to Ibutilide fumarate or any of the other product components.
4.4 Special warnings and precautions for use
Proarrhythmia
Like other antiarrhythmic agents, FIBRICOR Injection can induce or worsen ventricular arrhythmias in some patients. This may have potentially fatal consequences. Torsades de pointes, a polymorphic ventricular tachycardia that develops in the setting of a prolonged QT interval, may occur because of the effect Ibutilide has on cardiac repolarization, but Ibutilide can also cause polymorphic VT in the absence of excessive prolongation of the QT interval. In general, with drugs that prolong the QT interval, the risk of torsades de pointes is thought to increase progressively as the QT interval is prolonged and may be worsened with bradycardia, a varying heart rate, and hypokalemia.
In clinical trials conducted in patients with atrial fibrillation and atrial flutter, those with QTc intervals >440 msec were not usually allowed to participate, and serum potassium had to be above 4.0 mEq/L. Although change in QTc was dose dependent for Ibutilide, there was no clear relationship between risk of serious proarrhythmia and dose in clinical studies, possibly due to the small number of events. In clinical trials of intravenous Ibutilide, patients with a history of congestive heart failure (CHF) or low left ventricular ejection fraction appeared to have a higher incidence of sustained polymorphic ventricular tachycardia (VT), than those without such underlying conditions; for sustained polymorphic VT the rate was 5.4% in patients with a history of CHF and 0.8% without it. There was also a suggestion that women had a higher risk of proarrhythmia, but the sex difference was not observed in all studies and was most prominent for nonsustained ventricular tachycardia. The incidence of sustained ventricular arrhythmias was similar in male (1.8%) and female (1.5%) patients, possibly due to the small number of events. FIBRICOR is not recommended in patients who have previously demonstrated polymorphic ventricular tachycardia (eg, torsades de pointes).
During registration trials, 1.7% of patients with atrial flutter or atrial fibrillation treated with Ibutilide developed sustained polymorphic ventricular tachycardia requiring cardioversion. In these clinical trials, many initial episodes of polymorphic ventricular tachycardia occurred after the infusion of Ibutilide was stopped but generally not more than 40 minutes after the start of the first infusion.
There were, however, instances of recurrent polymorphic VT that occurred about 3 hours after the initial infusion. In two cases, the VT degenerated into ventricular fibrillation, requiring immediate defibrillation. Other cases were managed with cardiac pacing and magnesium sulfate infusions.
Nonsustained polymorphic ventricular tachycardia occurred in 2.7% of patients and nonsustained monomorphic ventricular tachycardias occurred in 4.9% of the patients.
Proarrhythmic events must be anticipated. Skilled personnel and proper equipment, including cardiac monitoring equipment, intracardiac pacing facilities, a cardioverter/defibrillator, and medication for treatment of sustained ventricular tachycardia, including polymorphic ventricular tachycardia, must be available during and after administration of FIBRICOR. Before treatment with FIBRICOR, hypokalemia and hypomagnesemia should be corrected to reduce the potential for proarrhythmia. Patients should be observed with continuous ECG monitoring for at least 4 hours following infusion or until QTc has returned to baseline. Longer monitoring is required if any arrhythmic activity is noted. Management of polymorphic ventricular tachycardia includes discontinuation of Ibutilide, correction of electrolyte abnormalities, especially potassium and magnesium, and overdrive cardiac pacing, electrical cardioversion, or defibrillation. Pharmacologic therapies include magnesium sulfate infusions. Treatment with antiarrhythmics should generally be avoided.
PRECAUTIONS
General
Antiarrhythmics
Class la antiarrhythmic drugs (Vaughan Williams Classification), such as disopyramide, quinidine, and procainamide, and other class III drugs, such as amiodarone and sotalol, should not be given concomitantly with Ibutilide Fumarate Injection or within 4 hours postinfusion because of their potential to prolong refractoriness. In the clinical trials, class I or other class III antiarrhythmic agents were withheld for at least 5 half-lives prior to Ibutilide infusion and for 4 hours after dosing, but thereafter were allowed at the physician's discretion.
Other drugs that prolong the QT interval
The potential for proarrhythmia may increase with the administration of Ibutilide Fumarate Injection to patients who are being treated with drugs that prolong the QT interval, such as phenothiazines, tricyclic antidepressants, tetracyclic antidepressants, and certain antihistamine drugs (H1 receptor antagonists).
Heart block
Of the nine (1.5%) Ibutilide-treated patients with reports of reversible heart block, five had first degree, three had second degree, and one had complete heart block.
Laboratory Test Interactions
None known.
4.5 Drug interactions
No specific pharmacokinetic or other formal drug interaction studies were conducted.
Digoxin
Supraventricular arrhythmias may mask the cardiotoxicity associated with excessive digoxin levels. Therefore, it is advisable to be particularly cautious in patients whose plasma digoxin levels are above or suspected to be above the usual therapeutic range. Co-administration of digoxin did not have effects on either the safety or efficacy of Ibutilide in the clinical trials.
Calcium channel blocking agents
Co-administration of calcium channel blockers did not have any effect on either the safety or efficacy of Ibutilide in the clinical trials.
Beta-adrenergic blocking agents
Co-administration of beta-adrenergic blocking agents did not have any effect on either the safety or efficacy of Ibutilide in the clinical trials.
4.6 Use in special populations
Pregnancy
Ibutilide administered orally was teratogenic (abnormalities included adactyly, interventricular septal defects, and scoliosis) and embryocidal in reproduction studies in rats. On a mg/m2 basis, corrected for the 3% oral bioavailability, the "no adverse effect dose" (5 mg/kg/day given orally) was approximately the same as the maximum recommended human dose (MRHD); the teratogenic dose (20 mg/kg/day given orally) was about four times the MRHD on a mg/m2 basis, or 16 times the MRHD on a mg/kg basis. Fibricore should not be administered to a pregnant woman unless clinical benefit outweighs potential risk to the fetus.
Breast-feeding
The excretion of ibutilide into breast milk has not been studied; accordingly, breastfeeding should be discouraged during therapy with Fibricor.
Pediatric use
Clinical trials with Ibutilide in patients with atrial fibrillation and atrial flutter did not include anyone under the age of 18. Safety and effectiveness of Ibutilide in pediatric patients has not been established.
Geriatric use
Clinical studies of Ibutilide Fumarate (involving 586 patients) did not include sufficient numbers of subjects less than age 65 (45%) to determine whether they respond differently from older subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Use in Patients With Hepatic or Renal Dysfunction:
The safety, effectiveness, and pharmacokinetics of Ibutilide Fumarate have not been established in patients with hepatic or renal dysfunction. However, it is unlikely that dosing adjustments would be necessary in patients with compromised renal or hepatic function based on the following considerations: (1) Ibutilide Fumarate is indicated for rapid intravenous therapy (duration ≤ 30 minutes) and is dosed to a known, well-defined pharmacologic action (termination of arrhythmia) or to a maximum of two 10-minute infusions; (2) less than 10% of the dose of Ibutilide Fumarate is excreted unchanged in the urine; and (3) drug distribution appears to be one of the primary mechanisms responsible for termination of the pharmacologic effect. Nonetheless, patients with abnormal liver function should be monitored by telemetry for more than the 4-hour period generally recommended.In 285 patients with atrial fibrillation or atrial flutter who were treated with Ibutilide Fumarate, the clearance of Ibutilide was independent of renal function, as assessed by creatinine clearance (range 21 to 140 mL/min).
4.7 Effects on the ability to drive and use machines
There are no known data available. Ability to drive and use machines may be affected by adverse reactions such as Headache.
4.8 Undesirable effects
Ibutilide Fumarate injection was generally well tolerated in clinical trials. Of the 586 patients with atrial fibrillation or atrial flutter who received Ibutilide in phase II/III studies, 149 (25%) reported medical events related to the cardiovascular system, including sustained polymorphic ventricular tachycardia (1.7%) and nonsustained polymorphic ventricular tachycardia (2.7%).
Other clinically important adverse events with an uncertain relationship to Ibutilide Fumarate include the following (0.2% represents one patient): sustained monomorphic ventricular tachycardia (0.2%), nonsustained monomorphic ventricular tachycardia (4.9%), AV block (1.5%), bundle branch block (1.9%), ventricular extrasystoles (5.1%), supraventricular extrasystoles (0.9%), hypotension/postural hypotension (2.0%), bradycardia/sinus bradycardia (1.2%), nodal arrhythmia (0.7%), congestive heart failure (0.5%), tachycardia/sinus tachycardia/supraventricular tachycardia (2.7%), idioventricular rhythm (0.2%), syncope (0.3%), and renal failure (0.3%). The incidence of these events, except for syncope, was greater in the group treated with Ibutilide Fumarate than in the placebo group.
Another adverse reaction that may be associated with the administration of Ibutilide Fumarate was nausea, which occurred with a frequency greater than 1% more in Ibutilide-treated patients than those treated with placebo.
The medical events reported for more than 1% of the placebo- and ibutilide-treated patients are shown in the following Table.
Treatment-Emergent Medical Events With Frequency of More Than 1% and Higher Than That of Placebo


In the post-cardiac surgery study, similar types of medical events were reported. In the 1 mg Ibutilide Fumarate treatment group (N=70), 2 patients (2.9%) developed sustained polymorphic ventricular tachycardia and 2 other patients (2.9%) developed nonsustained polymorphic ventricular tachycardia. Polymorphic ventricular tachycardia was not reported in the 73 patients in the 0.5 mg dose group or in the 75 patients in the 0.25 mg dose group.
Reporting of side effects
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Human Experience:
In the registration trials with Ibutilide Fumarate injection, four patients were unintentionally overdosed. The largest dose was 3.4 mg administered over 15 minutes. One patient (0.025 mg/kg) developed increased ventricular ectopy and monomorphic ventricular tachycardia, another patient (0.032 mg/kg) developed AV block—3rd degree and nonsustained polymorphic VT, and two patients (0.038 and 0.020 mg/kg) had no medical event reports. Based on known pharmacology, the clinical effects of an overdosage with ibutilide could exaggerate the expected prolongation of repolarization seen at usual clinical doses. Medical events (eg, proarrhythmia, AV block) that occur after the overdosage should be treated with measures appropriate for that condition.
5.0 Pharmacological Properties
5.1 Mechanism of Action
Ibutilide fumarate injection prolongs action potential duration in isolated adult cardiac myocytes and increases both atrial and ventricular refractoriness in vivo, i.e., class III electrophysiologic effects. Voltage clamp studies indicate that Ibutilide fumarate injection, at nanomolar concentrations, delays repolarization by activation of a slow, inward current (predominantly sodium). These effects lead to prolongation of atrial and ventricular action potential duration and refractoriness, the predominant electrophysiologic properties of Ibutilide fumarate injection in humans that are thought to be the basis for its antiarrhythmic effect.
5.2 Pharmacodynamic properties
Electrophysiologic Effects
Ibutilide Fumarate injection produces mild slowing of the sinus rate and atrioventricular conduction. Ibutilide Fumarate produces no clinically significant effect on QRS duration at intravenous doses up to 0.03 mg/kg administered over a 10-minute period. Although there is no established relationship between plasma concentration and antiarrhythmic effect, Ibutilide Fumarate produces dose-related prolongation of the QT interval, which is thought to be associated with its antiarrhythmic activity. In a study in healthy volunteers, intravenous infusions of Ibutilide Fumarate resulted in prolongation of the QT interval that was directly correlated with ibutilide plasma concentration during and after 10-minute and 8-hour infusions. A steep ibutilide concentration/response (QT prolongation) relationship was shown. The maximum effect was a function of both the dose of Ibutilide Fumarate and the infusion rate.
Hemodynamic Effects
A study of hemodynamic function in patients with ejection fractions both above and below 35% showed no clinically significant effects on cardiac output, mean pulmonary arterial pressure, or pulmonary capillary wedge pressure at doses of Ibutilide fumarate injection up to 0.03 mg/kg.
5.3 Pharmacokinetic properties
After intravenous infusion, Ibutilide plasma concentrations rapidly decrease in a multiexponential fashion. The pharmacokinetics of Ibutilide are highly variable among subjects. Ibutilide has a high systemic plasma clearance that approximates liver blood flow (about 29 mL/min/kg), a large steady-state volume of distribution (about 11 L/kg) in healthy volunteers, and minimal (about 40%) protein binding.
Ibutilide is also cleared rapidly and highly distributed in patients being treated for atrial flutter or atrial fibrillation. The elimination half-life averages about 6 hours (range from 2 to 12 hours). The pharmacokinetics of Ibutilide are linear with respect to the dose of Ibutilide Fumarate over the dose range of 0.01 mg/kg to 0.10 mg/kg. The enantiomers of Ibutilide fumarate have pharmacokinetic properties similar to each other and to Ibutilide fumarate.
The pharmacokinetics of Ibutilide Fumarate Injection in patients with atrial flutter or atrial fibrillation are similar regardless of the type of arrhythmia, patient age, sex, or the concomitant use of digoxin, calcium channel blockers, or beta blockers.
Metabolism and elimination:
In healthy male volunteers, about 82% of a 0.01 mg/kg dose of [14C] ibutilide fumarate was excreted in the urine (about 7% of the dose as unchanged ibutilide) and the remainder (about 19%) was recovered in the feces.
Eight metabolites of ibutilide were detected in metabolic profiling of urine. These metabolites are thought to be formed primarily by ω-oxidation followed by sequential β-oxidation of the heptyl side chain of ibutilide. Of the eight metabolites, only the ω-hydroxy metabolite possesses class III electrophysiologic properties similar to that of ibutilide in an in vitro isolated rabbit myocardium model. The plasma concentrations of this active metabolite, however, are less than 10% of that of ibutilide.
6.0 Nonclinical Properties
6.1 Animal Toxicology or Pharmacology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted to determine the carcinogenic potential of Ibutilide fumarate injection; however, it was not genotoxic in a battery of assays, (Ames assay, mammalian cell forward gene mutation assay, unscheduled DNA synthesis assay, and mouse micronucleus assay). Similarly, no drug-related effects on fertility or mating were noted in a reproductive study in rats in which Ibutilide was administered orally to both sexes up to doses of 20 mg/kg/day. On an mg/m2 basis, corrected for 3% bioavailability, the highest dose tested was approximately four times the maximum recommended human dose (MRHD).
7.0 Description
FIBRICOR Injection (Ibutilide fumarate injection) is an antiarrhythmic drug with predominantly class III (cardiac action potential prolongation) properties according to the Vaughan Williams Classification.
Ibutilide fumarate has one chiral center, and exists as a racemate of the (+) and (−) enantiomers.
Chemical Name: Methanesulfonamide, N-{4-{4-(ethylheptylamino)-1-hydroxybutyl}phenyl}, (+) (−), (E)-2-butenedioate (1:0.5) (hemifumarate salt)
Molecular Formula: C22H38N2O5S
Molecular Weight: 442.62 g/mol Structure:
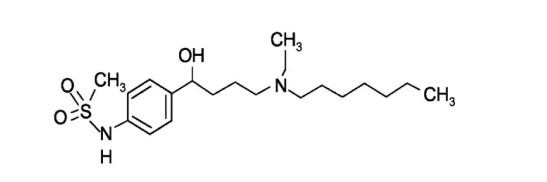
8.0 Pharmaceutical particulars
8.1 Incompatibilities
The following diluents are compatible with Ibutilide Fumarate injection (0.1 mg/mL):
5% Dextrose Injection
0.9% Sodium Chloride Injection
Admixtures of the product, with approved diluents, are chemically and physically stable for 24 hours at room temperature (15° to 30° C or 59° to 86° F)
Strict adherence to the use of aseptic technique during the preparation of the admixture is recommended in order to maintain sterility.
8.2 Shelf life
Refer on the pack.
8.3 Packaging Information
Each single dose vial contains Ibutilide Fumarate 0.1 mg/ml.
8.4 Storage and handling instructions
Store below 25°C. Do not freeze. Do not use in case any foreign particulate matter is observed inside the vial. Keep out of reach of children.
9.0 Patient Counselling Information
- FIBRICOR Injection can induce or worsen ventricular arrhythmias in some patients. Proarrhythmic events must be anticipated.
- Skilled personnel and proper equipment, including cardiac monitoring equipment, intracardiac pacing facilities, a cardioverter/defibrillator, and medication for treatment of sustained ventricular tachycardia, including polymorphic ventricular tachycardia, must be available during and after administration of FIBRICOR.
- Before treatment with FIBRICOR, hypokalemia and hypomagnesemia should be corrected to reduce the potential for proarrhythmia.
- Patients should be observed with continuous ECG monitoring for at least 4 hours following infusion or until QTc has returned to baseline. Longer monitoring is required if any arrhythmic activity is noted.
- Management of polymorphic ventricular tachycardia includes discontinuation of Ibutilide, correction of electrolyte abnormalities, especially potassium and magnesium, and overdrive cardiac pacing, electrical cardioversion, or defibrillation. Pharmacologic therapies include magnesium sulfate infusions. Treatment with antiarrhythmics should generally be avoided.
- Class la antiarrhythmic drugs (Vaughan Williams Classification), such as disopyramide, quinidine, and procainamide, and other class III drugs, such as amiodarone and sotalol, should not be given concomitantly with Ibutilide Fumarate Injection or within 4 hours postinfusion because of their potential to prolong refractoriness.
- The potential for proarrhythmia may increase with the administration of Ibutilide Fumarate Injection to patients who are being treated with drugs that prolong the QT interval, such as phenothiazines, tricyclic antidepressants, tetracyclic antidepressants, and certain antihistamine drugs (H1 receptor antagonists).
If you have any further questions, ask your doctor or pharmacist.
12.0 Date of revision of the text
This leaflet was last revised in October 2024.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1.What Fibricor is and what it is used for
2.What you need to know before you use Fibricor
3.How to use Fibricor
4.Possible side effects
5.How to store Fibricor
6.Contents of the pack and other information
1. What Fibricor is and what it is used for
Fibricor contains the active substance Ibutilide Fumarate, which belongs to a group of medicines called antiarrhythmics that help regulate your heart beat. FIBRICOR injection prolongs the action potential (change in the resting membrane potential of neurons and muscle cells) in heart cells and increases the time it takes for the heart's upper and lower chambers to be ready for another beat (ventricular refractoriness).
FIBRICOR is used:
For the rapid conversion of atrial fibrillation (irregular and rapid heartbeat) or atrial flutter (irregular heartbeat in the upper chambers of the heart) of recent onset to sinus rhythm (normal heart rhythm).
2. What you need to know before you use Fibricor
Do not use Fibricor
-If you are allergic to Ibutilide fumarate or any of the other ingredients of this medicine.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Fibricor if you have
- A history of heart failure or low left ventricular ejection fraction.
- Electrolyte imbalances (such as low potassium or magnesium levels).
- A prolonged QT interval on your ECG.
Children and adolescents
Fibricor is not recommended for use in children as it has not been proven to work in children below 18 years of age.
Other medicines and Fibricor
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
This is because Fibricor may influence the effectiveness of other medicines, so tell your doctor if you are taking:
- Medicines that prolong the QT interval, such as phenothiazines, tricyclic antidepressants, tetracyclic antidepressants, and certain antihistamine drugs (H1 receptor antagonists)
- Digoxin
- Calcium channel blocking agents
- Beta-adrenergic blocking agents
Pregnancy and breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. Fibricor should not be used during pregnancy unless clearly necessary. Breast-feeding should be discontinued during treatment with Fibricor.
Driving and using machines
Fibricor may affect your ability to drive and use machines. If you experience side effects like dizziness or headache, you should not drive or operate machines.
3. How to take Fibricor
Fibricor will be given to you by a healthcare professional in a hospital setting. The dose will depend on your body weight and the specific condition being treated. Continuous ECG monitoring is required during and after administration.
The recommended dose of FIBRICOR Injection based on controlled trials is outlined in the Table below:
Recommended Dose of FIBRICOR Injection
| Patient Weight | Initial Infusion (over 10 minutes) | Second Infusion |
| 60 kg or more Less than 60 kg | One vial (1 mg Ibutilide fumarate)0.1 mL/kg (0.01 mg/kg Ibutilide fumarate) | If the arrhythmia does not terminate within 10 minutes after the end of the initial infusion, a second 10-minute infusion of equal strength may be administered 10 minutes after completion of the first infusion. |
Continuous ECG monitoring is required during and after administration for at least 4 hours following infusion or until QTc has returned to baseline. Longer monitoring is required if any arrhythmic activity is noted.
Dilution
FIBRICOR Injection may be administered undiluted or diluted in 50 mL of diluent. FIBRICOR may be added to 0.9% Sodium Chloride Injection or 5% Dextrose Injection before infusion.
| Injection used for dilution→ | Ibutilide Injection 10 ml one Vial + 50 ml of 5% Dextrose Injection | Ibutilide Injection 10 ml one Vial + 50 ml of 0.9% Sodium Chloride Injection |
Use in Elderly patients
In general, dose selection for an elderly patient should be cautious.
Patients with liver problems
If you suffer from severe liver problems, no dosage adjustment required.
Patients with Kidney problems
No dose adjustment is necessary in patients with renal impairment.
Use in children and adolescents
These injections are not recommended for Use in children and adolescents under 18 years.
If you use more Fibricor than you should
These doses are carefully checked by your nurse or your doctor so an overdose is extremely unlikely. The known symptoms of overdose are increased ventricular ectopy (extra heartbeats originating in the ventricles), monomorphic ventricular tachycardia fast heartbeat from the lower chambers of the heart), AV block (delay or blockage in the electrical signals that control the heartbeat) and nonsustained polymorphic VT (Intermittent irregular fast heartbeat from the lower heart chambers that varies in shape).
If you have any further questions about the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you get any of the following side effects, tell your doctor immediately, or contact the casualty department at your nearest hospital:
Common side effects:
- Polymorphic ventricular tachycardia (Irregular fast heartbeat from the lower heart chambers that varies in shape)
- Nonsustained polymorphic ventricular tachycardia (Intermittent irregular fast heartbeat from the lower heart chambers that varies in shape)
- Headache
Other side effects:
- nonsustained monomorphic ventricular tachycardia (Temporary fast heartbeat originating from the lower chambers of the heart)
- AV block (delay or blockage in the electrical signals that control the heartbeat)
- bundle branch block (Blockage in the heart's electrical pathway)
- ventricular extrasystoles (Extra heartbeats from the lower chambers of the heart)
- QT prolongation (a change in the heart’s electrical activity)
- supraventricular extrasystoles (Extra heartbeats in the upper chambers of the heart.)
- hypotension/postural hypotension (Low blood pressure)
- bradycardia/sinus bradycardia (Slow heart rate)
- nodal arrhythmia (heart rhythm problem from the heart's node)
- congestive heart failure (heart not pumping well)
- tachycardia/sinus tachycardia/supraventricular tachycardia (Faster heart rate)
- idioventricular rhythm (heart rhythm that originates in the ventricles)
- syncope (fainting) renal failure (kidney failure)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly:
Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Fibricor
- Store below 25°C.
- Do not freeze.
- Do not use in case any foreign particulate matter is observed inside the vial.
- Keep out of reach of children.
- Refer pack insert for indications, dosage, precautions & instructions for reconstitution.
6. Contents of the pack and other information
What Fibricor contains
The active substance is Ibutilide fumarate. Each ml of solution contains 0.1 mg of ibutilide fumarate.
What Fibricor looks like and contents of the pack
Each ml contains: Ibutilide Fumarate……………………....0.1mg
(Equivalent to Ibutilide free base………0.087mg)
Water for Injections IP ……………. q.s.
Presentation/pack size
Each vial contains Ibutilide Fumarate 1 mg.
Presentation/pack size
Each vial contains Ibutilide Fumarate 1 mg.