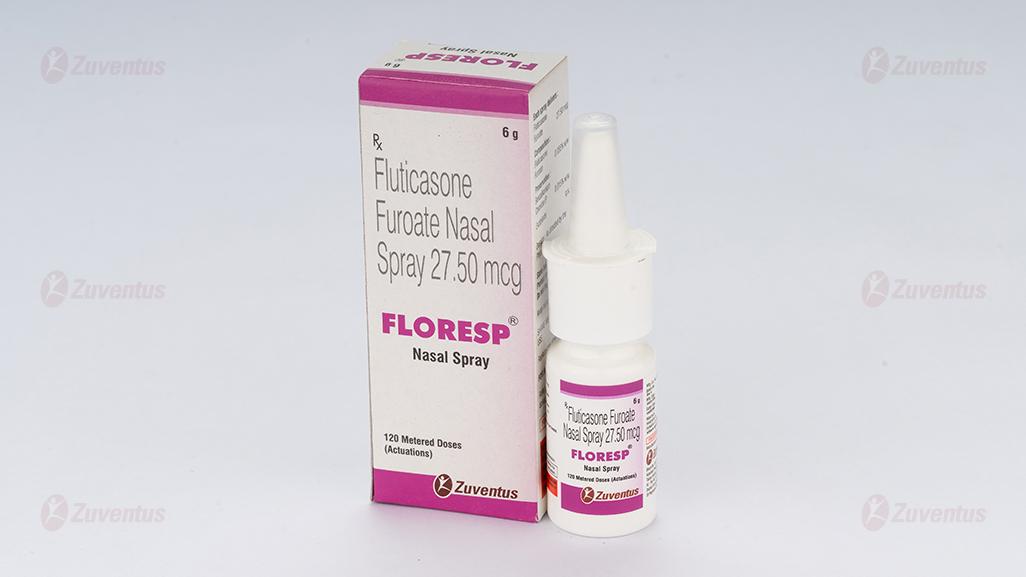
Floresp Nasal Spray
Therapy Area
Respiratory
1.0 Generic name
Fluticasone Furoate Nasal Spray 27.50 mcg
2.0 Qualitative and quantitative composition
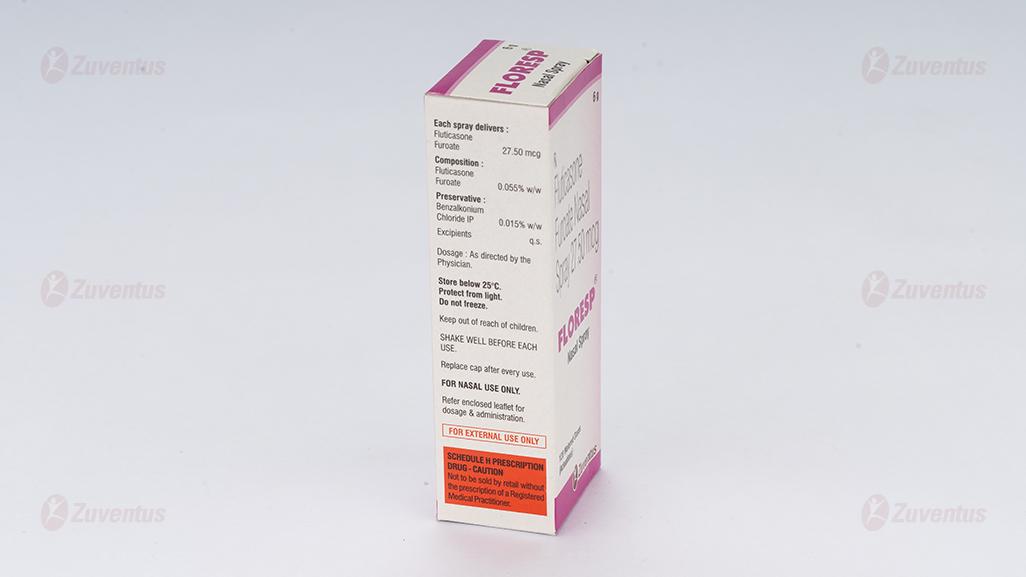
Each spray delivers :
Fluticasone Furoate EP 27.50 mcg
Composition :
Fluticasone Furoate EP 0.055 % w/w
Preservative :
Benzalkonium Chloride IP 0.015 % w/w
Excipients q.s.
3.0 Dosage form and strength
Nasal spray
4.0 Clinical particulars
4.1 Therapeutic indication
For the treatment of symptoms of allergic rhinitis.
4.2 Posology and method of administration
Adults and adolescents (12 years and over)
The recommended starting dose is two spray actuations (27.5 micrograms of fluticasone furoate per spray actuation) in each nostril once daily (total daily dose, 110 micrograms).
Once adequate control of symptoms is achieved, dose reduction to one spray actuation in each nostril (total daily dose 55 micrograms) may be effective for maintenance.
The dose should be titrated to the lowest dose at which effective control of symptoms is maintained.
Children (2 to 11 years of age)
The recommended starting dose is one spray actuation (27.5 micrograms of fluticasone furoate per spray actuation) in each nostril once daily (total daily dose, 55 micrograms).
Patients not adequately responding to one spray actuation in each nostril once daily (total daily dose, 55 micrograms) may use two spray actuations in each nostril once daily (total daily dose, 110 micrograms).
Once adequate control of symptoms is achieved, dose reduction to one spray actuation in each nostril once daily (total daily dose, 55 micrograms) is recommended.
For full therapeutic benefit regular, scheduled usage is recommended. Onset of action has been observed as early as 8 hours after initial administration. However, it may take several days of treatment to achieve maximum benefit, and the patient should be informed that their symptoms will improve with continuous regular use. The duration of treatment should be restricted to the period that corresponds to allergenic exposure.
Elderly Patients
No dose adjustment is required in this population.
Renal Impairment
No dose adjustment is required in this population.
Hepatic Impairment
No dose adjustment is required in patients with hepatic impairment.
Method of administration
Floresp nasal spray is for administration by the intranasal route only. The device should be cleaned after each use and the cap replaced.
Directions for use
Following are directions for the use of FLORESP nasal spray.
Parts of the nasal spray
As shown in figure 1, the bottle with pump system has nozzle (tip) for delivery of the spray in nostril and a protective dust cap.
Using your nasal spray
• STEP 1
Blow your nose gently and then remove the protective cap.
• STEP 2
Hold the bottle with your forefinger and middle finger on either side of the nozzle and your thumb underneath the bottle. (Figure 2)
• STEP 3
If using for the first time or if you have not used it for a week or more, test the spray. Testing the spray :With the nozzle pointing away from you, press down a few times until a fine mist comes out of the nozzle. (Figure 3)
• STEP 4
Close one nostril and hold the bottle as shown in figure 3. Tilt your head forward slightly and keeping the bottle upright, carefully insert the tip of the nozzle in the other nostril. (Figure 4)
• STEP 5
Start to breathe in through your nose and while breathing in, press down with your fingers once to release the spray. (Figure 4)
• STEP 6
Breathe out through your mouth.
• STEP 7
Repeat steps 4, 5 and 6 for the other nostril.
• STEP 8
Wipe the nozzle with a clean handkerchief/tissue and replace the protective dust cap. (Figure 5)

4.3 Contraindications
Hypersensitivity to the active substances or to any of the excipients.
4.4 Special warnings and precautions for use
Systemic corticosteroid effects
Systemic effects of nasal corticosteroid may occur, particularly at high doses prescribed for prolonged periods. These effects are much less likely to occur than with oral corticosteroids and may vary in individual patients and between different corticosteroid preparations. Potential systemic effects may include Cushing's syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, cataract, glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). Treatment with higher than recommended doses of nasal corticosteroids may result in clinically significant adrenal suppression. If there is evidence for higher than recommended doses being used, then additional systemic corticosteroid cover should be considered during periods of stress or elective surgery. Fluticasone furoate 110 micrograms once daily was not associated with hypothalamic-pituitary-adrenal (HPA) axis suppression in adult, adolescent or paediatric subjects. However, the dose of intranasal fluticasone furoate should be reduced to the lowest dose at which effective control of the symptoms of rhinitis is maintained. As with all intranasal corticosteroids, the total systemic burden of corticosteroids should be considered whenever other forms of corticosteroid treatment are prescribed concurrently.
If there is any reason to believe that adrenal function is impaired, care must be taken when transferring patients from systemic steroid treatment to fluticasone furoate.
Visual disturbance
Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient present with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
Growth retardation
Growth retardation has been reported in children receiving nasal corticosteroids at licensed doses. A reduction in growth velocity has been observed in children treated with fluticasone furoate 110 micrograms daily for one year. Therefore, children should be maintained on the lowest possible efficacious dose which delivers adequate symptom control. It is recommended that the growth of children receiving prolonged treatment with nasal corticosteroids is regularly monitored. If growth is slowed, therapy should be reviewed with the aim of reducing the dose of nasal corticosteroid if possible, to the lowest dose at which effective control of symptoms is maintained. In addition, consideration should be given to referring the patient to a paediatric specialist.
Patients on ritonavir
Concomitant administration with ritonavir is not recommended because of the risk of increased systemic exposure of fluticasone furoate.
4.5 Drugs interactions
Interaction with CYP3A inhibitors
Fluticasone furoate is rapidly cleared by extensive first pass metabolism mediated by the cytochrome P450 3A4.
Based on data with another glucocorticoid (fluticasone propionate), that is metabolised by CYP3A4, coadministration with ritonavir is not recommended because of the risk of increased systemic exposure of fluticasone furoate. Caution is recommended when co-administering fluticasone furoate with potent CYP3A inhibitors including cobicistat-containing products as an increase in the risk of systemic side effects is expected. Co-administration should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side effects, in which case patients should be monitored for systemic corticosteroid side effects. In a drug interaction study of intranasal fluticasone furoate with the potent CYP3A4 inhibitor ketoconazole there were more subjects with measurable fluticasone furoate concentrations in the ketoconazole group (6 of the 20 subjects) compared to placebo (1 out of 20 subjects). This small increase in exposure did not result in a statistically significant difference in 24-hour serum cortisol levels between the two groups. The enzyme induction and inhibition data suggest that there is no theoretical basis for anticipating metabolic interactions between fluticasone furoate and the cytochrome P450 mediated metabolism of other compounds at clinically relevant intranasal doses. Therefore, no clinical studies have been conducted to investigate interactions of fluticasone furoate on other drugs.
4.6 Use in special populations
Pregnancy
There are no adequate data from the use of fluticasone furoate in pregnant women. In animal studies glucocorticoids have been shown to induce malformations including cleft palate and intra-uterine growth retardation. This is not likely to be relevant for humans given recommended nasal doses which results in minimal systemic exposure. Fluticasone furoate should be used in pregnancy only if the benefits to the mother outweigh the potential risks to the foetus or child.
Nursing Mothers
It is unknown whether nasal administered fluticasone furoate is excreted in human breast milk.
Administration of fluticasone furoate to women who are breast-feeding should only be considered if the expected benefit to the mother is greater than any possible risk to the child.
Fertility
There are no fertility data in humans.
4.7 Effects on ability to drive and use machines
Floresp has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
The most commonly reported adverse reactions during treatment with fluticasone furoate are epistaxis, nasal ulceration and headache. The most serious undesirable effects are rare reports of hypersensitivity reactions, including anaphylaxis (less than 1 case per 1000 patients). The following convention has been used for the classification of frequencies : Very common ≥1/10; Common ≥1/100 to <1/10; Uncommon ≥1/1000 to <1/100; Rare ≥1/10,000 to <1/1000; Very rare <1/10,000; Not known (cannot be estimated from the available data).
• Immune system disorders : Rare- Hypersensitivity reactions including anaphylaxis, angioedema, rash, and urticaria.
• Nervous system disorders : Common- Headache.
• Eye disorders : Not known- Transient ocular changes, vision blurred.
• Respiratory, thoracic and mediastinal disorders : Very common- *Epistaxis, Common- Nasal ulceration, dyspnoea**, Uncommon- Rhinalgia, nasal discomfort (including nasal burning, nasal irritation, and nasal soreness), nasal dryness. Very rare- Nasal septum perforation, Not known- Bronchospasm.
• Musculoskeletal and connective tissue disorders (Children) : Not known- ***Growth retardation (see Clinical experience).
Description of selected adverse reactions
Epistaxis
*Epistaxis was generally mild to moderate in intensity. In adults and adolescents, the incidence of epistaxis was higher in longer-term use (more than 6 weeks) than in short-term use (up to 6 weeks).
Systemic effects
Systemic effects of nasal corticosteroids may occur, particularly when prescribed at high doses for prolonged periods. Growth retardation has been reported in children receiving nasal corticosteroids.
**Dyspnoea cases were reported in more than 1% of patients during clinical trials with fluticasone furoate; similar rates were also observed in placebo groups.
Paediatric population
The safety in children under 6 years has not been well established. Frequency, type and severity of adverse reactions observed in the paediatric population are similar to those in the adult population.
Epistaxis
*In paediatric clinical studies of up to 12 weeks duration the incidence of epistaxis was similar between patients receiving fluticasone furoate and patients receiving placebo.
Growth retardation
***In a one-year clinical study assessing growth in pre-pubescent children receiving 110 micrograms of fluticasone furoate once daily, an average treatment difference of -0.27 cm per year in growth velocity was observed compared to placebo (see Clinical efficacy and safety).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com Website : https://www.zuventus.co.in/drug-safety-reporting
4.9 Overdose
In a bioavailability study, intranasal doses of up to 2640 micrograms per day were administered over three days with no adverse systemic reactions observed.
Acute overdose is unlikely to require any therapy other than observation.
5.0 Pharmacological properties
5.1 Mechanism of action
Fluticasone furoate is a synthetic trifluorinated corticosteroid that possesses a very high affinity for the glucocorticoid receptor and has a potent anti-inflammatory action.
5.2 Pharmacodynamic properties
Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in inflammation. Specific effects of fluticasone furoate demonstrated in in vitro and in vivo models included activation of the glucocorticoid response element, inhibition of pro-inflammatory transcription factors such as NFkB, and inhibition of antigen-induced lung eosinophilia in sensitized rats.
Fluticasone furoate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is approximately 29.9 times that of dexamethasone and 1.7 times that of fluticasone propionate. The clinical relevance of these findings is unknown.
5.3 Pharmacokinetic properties
Absorption : Fluticasone furoate undergoes incomplete absorption and extensive first-pass metabolism in the liver and gut resulting in negligible systemic exposure. The intranasal dosing of 110 micrograms once daily does not typically result in measurable plasma concentrations (<10 pg/mL). The absolute bioavailability for intranasal fluticasone furoate is 0.50 %, such that less than 1 microgram of fluticasone furoate would be systemically available after administration of 110 micrograms.
Distribution : The plasma protein binding of fluticasone furoate is greater than 99 %. Fluticasone furoate is widely distributed with volume of distribution at steady-state of, on average, 608 L.
Biotransformation : Fluticasone furoate is rapidly cleared (total plasma clearance of 58.7 L/h) from systemic circulation principally by hepatic metabolism to an inactive 17β-carboxylic metabolite (GW694301X), by the
cytochrome P450 enzyme CYP3A4. The principal route of metabolism was hydrolysis of the S-fluoromethyl carbothioate function to form the 17β- carboxylic acid metabolite. In vivo studies have revealed no evidence of cleavage of the furoate moiety to form fluticasone. Elimination : Elimination was primarily via the faecal route following oral and intravenous administration indicative of excretion of fluticasone furoate and its metabolites via the bile. Following intravenous administration, the elimination phase half-life averaged 15.1 hours. Urinary excretion accounted for approximately 1 % and 2 % of the orally and intravenously administered dose, respectively.
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, pharmacist or nurse.
This medicine has been prescribed for you only.
Do not pass it on to others.
It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor, pharmacist or nurse.
This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
What FLORESP is and what it is used for
What you need to know before you take FLORESP
How to take FLORESP
Possible side effects
How to store FLORESP
Contents of the pack and other information
1. What Floresp is and What It is Used for
FLORESP (fluticasone furoate) belongs to a group of medicines called glucocorticoids. FLORESP works to decrease inflammation caused by allergy (rhinitis) and therefore reduce symptoms of allergy.
FLORESP nasal spray is used to treat symptoms of allergic rhinitis including stuffy, runny or itchy nose, sneezing and watery, itchy or red eyes, in adults and children aged 2 years and over.
Allergy symptoms can occur at specific times of the year and be caused by allergy to pollen from grass or trees (hayfever), or they can occur all year round and be caused by allergy to animals, house-dust mites or moulds to name some of the most common.
2. What You Need to Know Before You Take Floresp
Do not use FLORESP
If you are allergic to fluticasone furoate or any of the other ingredients of this medicine
Warnings and precautions
Children and adolescents
Do not use in children under 2 years old.
Taking FLORESP:
- may when taken for a long time cause children to grow more slowly. The doctor will check your child’s height regularly, and make sure he or she is taking the lowest possible effective dose.
- may cause eye conditions such as glaucoma (increase in pressure in the eye) or cataracts (clouding of the lens of the eye). Tell your doctor if you had these conditions in the past, or if you notice blurred vision or other visual disturbances while you are taking FLORESP.
Other medicines and FLORESP
Tell your doctor or pharmacist if you are taking, or have recently taken, or might take any other medicines, including medicines obtained without a prescription.
It is especially important to tell your doctor if you are taking, or have recently taken any of the following medicines:
- steroid tablets or injected steroids
- steroid creams
- medicines for asthma
- ritonavir or cobicistat, used to treat HIV
- ketoconazole, used to treat fungal infections
Your doctor will assess whether you should take FLORESP with these medicines. Your doctor may wish to monitor you carefully if you are taking any of these medicines as they may increase the side effects of FLORESP.
FLORESP should not be used at the same time with other nasal sprays containing steroids.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Do not use FLORESP if you are pregnant, or planning to become pregnant, unless your doctor or pharmacist tells you to.
Do not use FLORESP if you are breast feeding unless your doctor or pharmacist tells you to.
Driving and using machines
FLORESP is unlikely to affect your ability to drive and use machines.
FLORESP contains benzalkonium chloride
This medicine contains benzalkonium chloride. Benzalkonium chloride may cause irritation or swelling inside of the nose, especially if used for a long time. Tell your doctor or pharmacist if you feel discomfort when using the spray.
3. How to Use Floresp
Always use this medicine exactly as your doctor or pharmacist has told you. Don’t exceed the recommended dose. Check with your doctor or pharmacist if you’re not sure.
When to use FLORESP
Use once a day
Use at the same time each day.
This will treat your symptoms throughout the day and night.
How long FLORESP takes to work
Some people will not feel the full effects until several days after first using FLORESP. However, it is usually effective within 8 to 24 hours of use.
How much to use
Adults and children 12 years and over
The usual starting dose is 2 sprays in each nostril once every day.
Once symptoms are controlled you may be able to decrease your dose to 1 spray in each nostril, once every day.
Children 2 to 11 years
The usual starting dose is 1 spray in each nostril once a day.
If symptoms are very bad your doctor may increase the dose to 2 sprays in each nostril once every day until the symptoms are under control. It may then be possible for the dose to be reduced to 1 spray in each nostril once every day.
How to use the nasal spray
Be careful not to get any spray into your eyes. If you do, rinse your eyes with water. There is a step-by-step guide to using the nasal spray after Section 6 of this leaflet. Follow the guide carefully to get full benefit from using FLORESP.
If you take more FLORESP than you should
talk to a doctor or pharmacist immediately
If you forget to take FLORESP
If you miss a dose, take it when you remember. If it is nearly the time for your next dose, wait until then. Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, or if you have any discomfort using the nasal spray ask your doctor or pharmacist or nurse.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions: get a doctor’s help straight away
Allergic reactions to FLORESP are rare and affect less than 1 person in 1,000. In a small number of people, allergic reactions can develop into a more serious, even life-threatening problem if not treated. Symptoms include:
- becoming very wheezy, coughing or having difficulty with breathing
- suddenly feeling weak or light-headed (which may lead to collapse or loss of consciousness)
- swelling around the face
- skin rashes or redness.
In many cases, these symptoms will be signs of less serious side effects. But you must be aware that they are potentially serious — so, if you notice any of these symptoms: Contact a doctor as soon as possible.
Very common side effects (may affect more than 1 in 10 people)
Nosebleeds (generally minor), particularly if you use FLORESP for more than 6 weeks continuously.
Common side effects (may affect up to 1 in 10 people)
- Nasal ulceration – which may cause irritation or discomfort in your nose. You may also get streaks of blood when you blow your nose.
- Headache.
- Shortness of breath
Uncommon side effects (may affect up to 1 in 100 people)
Pain, burning, irritation, soreness or dryness in the inside of the nose.
Very rare side effects (may affect up to 1 in 10,000 people)
Small holes (perforations) in the ridge inside the nose that separates the nostrils.
Not known (frequency cannot be estimated from the available data)
- Slowing of growth in children.
- Blurred vision or temporary changes to vision with long term use.
- Chest tightness causing difficulty in breathing.
Nasal corticosteroids can affect the normal production of hormones in your body, particularly if you use high doses for a long time. In children this side effect can cause them to grow more slowly than others.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to Store Floresp
Store below 25°C. Protected from light. Do not freeze.
Keep out of reach of children.
SHAKE WELL BEFORE EACH USE.
FOR NASAL USE ONLY.
Refer enclosed leaflet for dosage & administration.
6. Contents of the Pack and Other Information
What FLORESP contains
Each spray delivers:
Fluticasone Furoate 27.50 mcg
Composition:
Fluticasone Furoate 0.055% w/w
Preservative:
Benzalkonium Chloride IP 0.015% w/w Excipients q.s.
DIRECTIONS FOR USE
Following are directions for the use of FLORESP nasal spray.
PARTS OF THE NASAL SPRAY
As shown in Figure 1, the bottle with pump system has nozzle (tip) for delivery of the spray in nostril and a protective dust cap.
USING YOUR NASAL SPRAY
STEP 1
Blow your nose gently and then remove the protective cap.
STEP 2
Hold the bottle with your forefinger and middle finger on either side of the nozzle and your thumb underneath the bottle (Figure 2).
STEP 3
If using for the first time or if you have not used it for a week or more, test the spray. Testing the spray: With the nozzle pointing away from you, press down a few times until a fine mist comes out of the nozzle. (Figure 3)
STEP 4
Close one nostril and hold the bottle as shown in figure 3. Tilt your head forward slightly and keeping the bottle upright, carefully insert the tip of the nozzle in the other nostril. (Figure 4)
STEP 5
Start to breathe in through your nose and while breathing in, press down with your fingers once to release the spray. (Figure 4)
STEP 6
Breathe out through your mouth.
STEP 7
Repeat steps 4, 5 and 6 for the other nostril.
STEP 8
Wipe the nozzle with a clean handkerchief/tissue and replace the protective dust cap.(Figure 5)
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Findings in general toxicology studies were similar to those observed with other glucocorticoids and are associated with exaggerated pharmacological activity. These findings are not likely to be relevant for humans given recommended nasal doses which results in minimal systemic exposure. No genotoxic effects of fluticasone furoate have been observed in conventional genotoxicity tests. Further, there were no treatment-related increases in the incidence of tumours in two year inhalation studies in rats and mice.
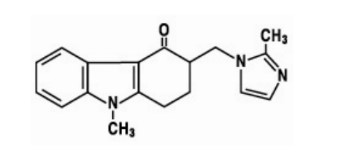
7.0 Description
Fluticasone furoate, the active component of floresp Nasal Spray, is a synthetic fluorinated corticosteroid having the chemical name (6α,11β,16α,17α)-6,9- difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3- oxoandrosta-1,4-dien-17-yl 2-furancarboxylate.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
FLORESP sales pack contains120 metered doses.
8.4 Storage and handling instructions
Store below 25°C. Protected from light. Do not freeze.
Keep out of reach of children. SHAKE WELL BEFORE EACH USE. FOR NASAL USE ONLY. Refer enclosed leaflet for dosage & administration.
9.0 Patient counselling information
• Local Nasal Effects : Patients should be informed that treatment with Floresp Nasal Spray may lead to adverse reactions, which include epistaxis and nasal ulceration. Candida infection may also occur with treatment with Floresp Nasal Spray. In addition, nasal corticosteroids are associated with nasal septal perforation and impaired wound healing. Patients who have experienced recent nasal ulcers, nasal surgery, or nasal trauma should not use Floresp Nasal Spray until healing has occurred
• Cataracts and Glaucoma : Patients should be informed that glaucoma and cataracts are associated with nasal and inhaled corticosteroid use. Patients should inform his/her health care provider if a change in vision is noted while using Floresp Nasal Spray
• Hypersensitivity Reactions, Including Anaphylaxis : Patients should be aware that hypersensitivity reactions, including anaphylaxis, angioedema, rash, and urticaria, may occur after administration of Floresp Nasal Spray. If such reactions occur, patients should discontinue use of Floresp Nasal Spray
• Immunosuppression : Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay. Patients should be informed of potential worsening of existing tuberculosis, fungal, bacterial, viral or parasitic infections, or ocular herpes simplex
• Use Daily for Best Effect : Patients should use Floresp Nasal Spray on a regular once-daily basis for optimal effect. Floresp Nasal Spray, like other corticosteroids, does not have an immediate effect on rhinitis symptoms. Although significant improvement is usually achieved within 24 hours in patients with seasonal allergic rhinitis and 4 days in patients with perennial allergic rhinitis, maximum benefit may not be reached for several days. The patient should not increase the prescribed dosage but should contact the physician if symptoms do not improve or if the condition worsens.
• Keep Spray Out of Eyes : Patients should be informed to avoid spraying Floresp Nasal Spray in their eyes.
• Potential Drug Interactions : Patients should be advised that coadministration of Floresp Nasal Spray and ritonavir is not recommended and to be cautious if coadministrating with ketoconazole.
• Do not take this medicine, if you are allergic to Fluticasone furoate.
• Signs of an allergic reaction include a rash, itching or shortness of breath.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
• If you have any further questions, ask your doctor or pharmacist.
12.0 Date of revision
11 July 2024