KIMET XL 12.5 Tablets
Therapy Area
Cardiology
1.0 Generic name
S (-) Metoprolol Succinate Prolonged-Release Tablets
2.0 Qualitative and quantitative composition
KIMET XL®-12.5
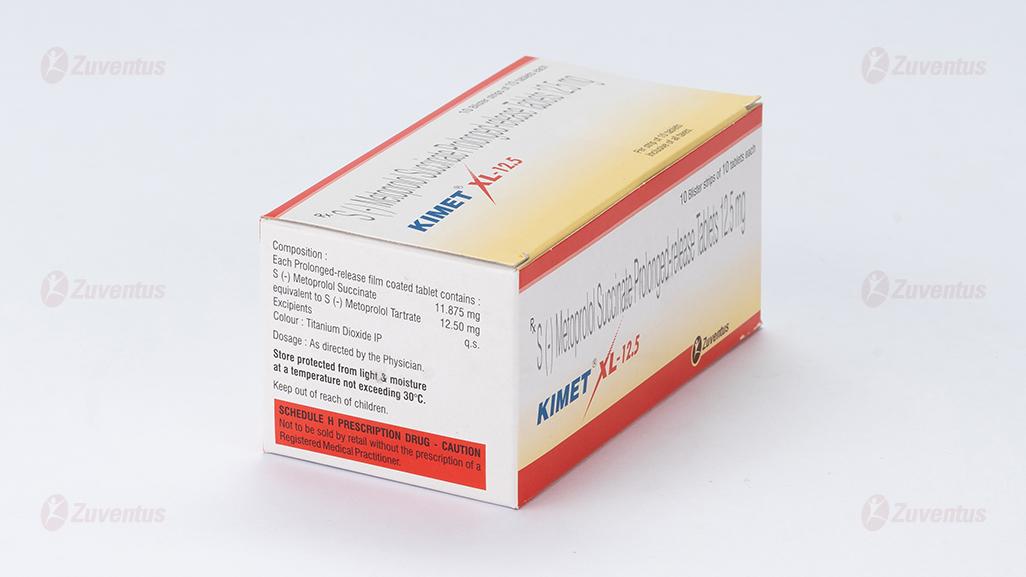
Each prolonged-release film-coated tablet contains:
S (-) Metoprolol Succinate 11.875 mg
equivalent to S (-) Metoprolol Tartrate 12.50 mg
Colour: Titanium Dioxide IP
KIMET XL®-25
Each prolonged-release film-coated tablet contains:
S (-) Metoprolol Succinate 23.75 mg
equivalent to S (-) Metoprolol Tartrate 25 mg
Colour: Titanium Dioxide IP
KIMET XL®-50
Each prolonged-release film-coated tablet contains
S (-) Metoprolol Succinate 47.50 mg
equivalent to S (-) Metoprolol Tartrate 50 mg
Colour: Titanium Dioxide IP
3.0 Dosage form and strength
Tablets, 12.5 mg, 25 mg, 50 mg
4.0 Clinical particulars
4.1 Therapeutic indication
KIMET XL® tablets are indicated for the treatment of hypertension and angina pectoris.
KIMET XL® tablets are also used for the treatment of functional heart disorders, migraine prophylaxis, cardiac arrhythmias, prevention of cardiac death and re-infarction after the acute phase of myocardial infarction stable symptomatic CHF.
4.2 Posology and method of administration
One KIMET XL® tablet daily treatment is preferably taken in the morning. The dosage of KIMET XL® tablets should be individualized.
Hypertension: The usual initial dosage of KIMET XL® is 12.5 to 50 mg daily in a single dose alone or combined with other antihypertensive agents. A further reduction in blood pressure may be achieved if S (-) Metoprolol is used in conjunction with an antihypertensive diuretic or other hypotensive agent.
Angina Pectoris: KIMET XL® 25-50 mg twice or three times daily. In general, a significant improvement in exercise tolerance and reduction of anginal attacks may be expected with a dose of 25-50 mg twice daily.
Cardiac Arrhythmias: KIMET XL® dosage of 25 mg two or three times daily is usually sufficient. If necessary, the dose can be increased up to 150 mg per day and administered in divided doses.
Myocardial infarction: Early intervention: 25 mg every 6 hours for 48 hours, preferably within 12 hours of the onset of chest pain. Maintenance: The usual maintenance dose of KIMET XL® is 100 mg daily given in divided doses. The treatment should be continued for at least 3 months.
Prophylaxis of migraine: KIMET XL® dosage of 50-100 mg daily, given in divided doses (morning and evening).
Stable heart failure, function class II and III: A recommended initial dosage of KIMET XL® for the first two weeks is 12.5 mg once daily. In patients with more severe heart failure, the starting dose is 6.25mg once daily. After two weeks, the dosage can be doubled every second week upto the dosage tolerated by the patient. The target dose for long-term treatment is 100 mg once daily.
4.3 Contraindications
Hypersensitivity to the active substances, to related derivatives or to any of the excipients. KIMET XL® is contraindicated in
- Severe asthma or history of severe bronchospasm.
- Atrioventricular block of second or third degree.
- Uncontrolled heart failure.
- Clinically relevant sinus bradycardia.
- Sick-sinus syndrome.
- Severe peripheral arterial disease.
- Cardiogenic shock.
- Hypotension.
- Untreated phaeochromocytoma.
- Metabolic acidosis.
KIMET XL® is also contraindicated when myocardial infarction is complicated by significant bradycardia, first-degree heart block, systolic hypotension (less than 100 mmHg) and/or severe heart failure.
4.4 Special warnings and precautions for use
Special warnings
Cardiac Failure: In hypertensive and angina patients who have congestive heart failure controlled by digitalis and diuretics, KIMET XL® should be administered cautiously as β blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe failure.
In Patients without a History of Cardiac Failure: Continued depression of the myocardium with β-blocking agents over a period of time can, in some cases, lead to cardiac failure.
Ischemic Heart Disease: Following abrupt cessation of therapy with certain β-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction has occurred. When discontinuing chronically administered KIMET XL®, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1-2 weeks and the patient should be carefully monitored.
Bronchospastic Diseases: Patients with bronchospastic diseases should, in general, not receive β blockers, including KIMET XL®. Because of its relative β1 selectivity, however, KIMET XL may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since β1 selectivity is not absolute, a β2-stimulating agent should be administered concomitantly, and the lowest possible dose of KIMET XL® should be used. In these circumstances it would be prudent initially to administer KIMET XL® in smaller doses three times daily, instead of larger doses two times daily, to avoid the higher plasma levels associated with the longer dosing interval.
Major Surgery: Chronically administered β-blocking therapy should not be routinely withdrawn prior to major surgery.
Diabetes and Hypoglycemia: β blockers may mask tachycardia occurring with hypoglycemia.
Pheochromocytoma: If KIMET XL® is used in the setting of pheochromocytoma, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of β blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of β-mediated vasodilatation in skeletal muscle.
Thyrotoxicosis: β-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of β blockade, which might precipitate a thyroid storm.
Bradycardia: KIMET XL® produces a decrease in sinus heart rate in most patients. Acute myocardial infarction (particularly inferior infarction) may in itself produce significant lowering of the sinus rate. If the sinus rate decreases to <40 beats/min, particularly if associated with evidence of lowered cardiac output, atropine (0.25-0.5 mg) should be administered intravenously.
AV Block: KIMET XL® slows AV conduction and may produce significant first- (P-R interval≥0.26 sec), second-, or third-degree heart block. If heart block occurs, KIMET XL® should be discontinued and atropine (0.25-0.5 mg) should be administered intravenously.
Hypotension: If hypotension (systolic blood pressure ≤90 mmHg) occurs, KIMET XL® should be discontinued, and the hemodynamic status of the patient and the extent of myocardial damage carefully assessed.
Bronchospastic Diseases: Patients with bronchospastic diseases should, in general, not receive β blockers, including KIMET XL. Because of its relative β1 selectivity, KIMET XL® may be used with extreme caution in patients with bronchospastic disease.
Precautions
KIMET XL® should be used with caution in patients with impaired hepatic function.
KIMET XL® should be taken regularly and continuously as directed. It should not be discontinued without consulting the physician. Abrupt interruption of the medication is to be avoided. Sudden withdrawal of β blockade is hazardous, especially in high risk patients, and may aggravate chronic heart failure as well as increase the risk of myocardial infarction and sudden death. Any withdrawal of the product should therefore, if possible, be made gradually over at least two weeks where the dose is reduced by half in each step, down to the final dose when a 25 mg tablet is reduced to half a tablet. The final dose should be given for at least four days before discontinuation.
It is advisable (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with KIMET XL® has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking KIMET XL®.
Concomitant use of KIMET XL® with catecholamine-depleting drugs or digitalis glycosides can increase the risk of bradycardia and hypotension.
Risk of Anaphylactic Reaction: While taking β blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated challenges, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
General Anesthetics: Some inhalation anesthetics may enhance the cardio-depressant effect of β blockers.
CYP2D6 Inhibitors: Potent inhibitors of the CYP2D6 enzyme may increase the plasma concentration of KIMET XL®. Caution should therefore be exercised when co-administering potent CYP2D6 inhibitors with KIMET XL®.
Clonidine: If a patient is treated with clonidine and KIMET XL® concurrently, and clonidine treatment is to be discontinued, KIMET XL® should be stopped several days before clonidine is withdrawn.
4.5 Drugs interactions
The effects of S (-) Metoprolol and other antihypertensive drugs on blood pressure are usually additive, and care should be taken to avoid hypotension. However, combinations of antihypertensive drugs may often be used with the benefit to improve the control of hypertension.
As beta-blockers may affect peripheral circulation, care should be exercised when drugs with similar activity, e.g. ergotamine are given concurrently.
Care should also be exercised when beta-blockers are given in combination with sympathetic ganglion-blocking agents, other beta-blockers (also in the form of eye drops), or MAO inhibitors.
Prazosin
The acute postural hypotension that can follow the first dose of prazosin may be increased in patients already taking a beta-blocker.
Clonidine
If combination treatment with clonidine is to be discontinued S (-) Metoprolol should be withdrawn several days before clonidine. This is because the hypertension that can follow withdrawal of clonidine may be increased in patients receiving concurrent beta-blocker treatment.
Calcium channel blockers
Calcium channel blockers such as verapamil and diltiazem may potentiate the depressant effects of beta-blockers on blood pressure, heart rate, cardiac contractility and atrioventricular conduction. A calcium channel blocker of the verapamil (phenylalkylamine) type should not be given intravenously to patients receiving S (-) Metoprolol because there is a risk of cardiac arrest in this situation. Patients taking an oral calcium channel blocker of the verapamil type in combination with S (-) Metoprolol should be closely monitored.
CYP2D6 inhibitors
Potent inhibitors of this enzyme may increase the plasma concentration of S (-) Metoprolol (see section 5.2). Caution should therefore be exercised when co-administering potent CYP2D6 inhibitors with metoprolol. Known clinically significant potent inhibitors of CYP2D6 are antidepressants such as fluoxetine, paroxetine or bupropion, antipsychotics such as thioridazine, antiarrhythmics such as propafenone, antiretrovirals such as ritonavir, antihistamines such as diphenhydramine, antimalarials such as hydroxychloroquine or quinidine, antifungals such as terbinafine and medications for stomach ulcers such as cimetidine.
Class I anti-arrhythmic drugs and amiodarone
Amiodarone, propafenone, and other class I anti-arrhythmic agents such as quinidine and disopyramide may potentiate the effects of beta-blockers on heart rate and atrioventricular conduction.
Nitroglycerin
Nitroglycerin may enhance the hypotensive effect of metoprolol.
Digitalis glycosides
Concurrent use of digitalis glycosides may result in excessive bradycardia and/or increase in atrioventricular conduction time.
Sympathomimetics
S (-) Metoprolol will antagonise the beta1 effects of sympathomimetic agents but should have little influence on the bronchodilator effects of beta2-agonists at normal therapeutic doses.
Insulin and oral hypoglycaemic drugs
In diabetic patients who use insulin, beta-blocker treatment may be associated with increased or prolonged hypoglycaemia. Beta-blockers may also antagonize the hypoglycaemic effects of sulfonylureas. The risk of either effect is less with a beta1-selective drug such as S (-) Metoprolol than with a non-selective beta-blocker. However, diabetic patients receiving S (-) Metoprolol should be monitored to ensure that diabetes control is maintained.
Non-steroidal anti-inflammatory drugs
Concurrent treatment with non-steroidal anti-inflammatory drugs such as indomethacin may decrease the antihypertensive effect of metoprolol.
Lignocaine
S (-) Metoprolol may impair the elimination of lignocaine.
General anaesthetics
Some inhalation anaesthetics may enhance the cardio-depressant effect of beta-blockers.
Hepatic enzyme inducers/inhibitors
Enzyme-inducing agents (e.g. rifampicin) may reduce plasma concentrations of metoprolol, whereas enzyme inhibitors (e.g. cimetidine) may increase plasma concentrations.
Alcohol
During concomitant ingestion of alcohol and metoprolol, the concentration of blood alcohol may reach higher levels and may decrease more slowly.
4.6 Use in special populations
S(-)Metoprolol should not be used in pregnancy or lactation unless it is considered that the benefit outweighs the possible risk to the fetus/infant.
If S(-)Metoprolol is used during pregnancy and lactation special attention should be paid to the fetus, neonate and breastfed infant for undesirable effects of the drug's beta-blocking action (e.g. bradycardia, hypoglycemia). The lowest possible dose should be used, and treatment should be discontinued at least 2 to 3 days before delivery to avoid increased uterine contractility and the effects of beta-blockade in the newborn baby.
Pregnancy
Beta-blockers reduce placental perfusion which may result in intrauterine fetal death, and immature and premature deliveries.
S(-)Metoprolol has, however, been used in pregnancy-associated hypertension under close supervision after 20 weeks gestation. Although the drug crosses the placental barrier and is present in cord blood no evidence of fetal abnormalities has been reported. Animal experiments have shown neither teratogenic potential nor other adverse events on the embryo and/or fetus relevant to the safety assessment of the product.
Breast-feeding
The amount of S(-)Metoprolol ingested via breast milk seems to be negligible with regard to its beta-blocking effects if the mother is treated in doses within the therapeutic range.
4.7 Effects on ability to drive and use machines
As with all beta-blockers, S(-)Metoprolol may affect patients' ability to drive and operate machinery. Patients should be warned accordingly.
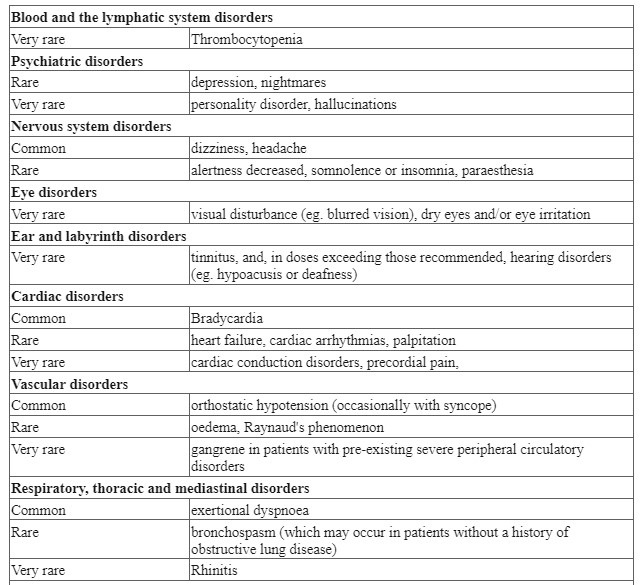
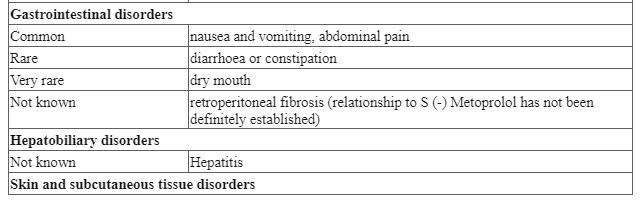
4.8 Undesirable effects
Adverse reactions have been ranked under headings of frequency using the following convention: very common (≥ 1/10); common (≥ 1/100 to <1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000); not known.
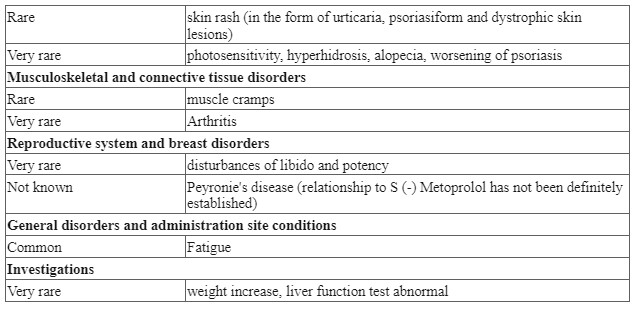
Post-marketing experience
The following adverse reactions have been reported during post-approval use of S(-)Metoprolol: a confusional state, an increase in blood triglycerides and a decrease in high-density lipoprotein (HDL). Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
4.9 Overdose
Symptoms
In more severe cases an overdosage of S(-)Metoprolol may lead to severe hypotension, sinus bradycardia, atrioventricular block, heart failure, cardiogenic shock, cardiac arrest, bronchospasm, impairment of consciousness, coma, convulsions, nausea, vomiting, cyanosis, hypoglycaemia and occasionally hyperkalaemia.
Management
Patients should be admitted to hospital and, generally, should be managed in an intensive care setting, with continuous monitoring of cardiac function, blood gases, and blood biochemistry. Emergency supportive measures such as artificial ventilation or cardiac pacing should be instituted if appropriate. Even apparently well patients who have taken a small overdose should be closely observed for signs of poisoning for at least 4 hours. In the event of a potentially life-threatening oral overdose, use induction of vomiting or gastric lavage (if within 4 hours after ingestion of metoprolol) and/or activated charcoal to remove the drug from the gastrointestinal tract. S (-) Metoprolol cannot be effectively removed by haemodialysis.
Atropine may be given intravenously to control significant bradycardia. Intravenous beta-agonists such as prenalterol or isoprenaline should be used to treat bradycardia and hypotension; very high doses may be needed to overcome the beta blockade. Dopamine, dobutamine or noradrenaline may be given to maintain blood pressure. Glucagon has positive inotropic and chronotropic effects on the heart that are independent of beta-adrenergic receptors and has proved effective in the treatment of resistant hypotension and heart failure associated with beta-blocker overdose.
5.0 Pharmacological properties
5.1 Mechanism of Action
S(-)Metoprolol has a relatively greater blocking effect on beta1-receptors (i.e. those mediating adrenergic stimulation of heart rate and contractility and release of free fatty acids from fat stores) than on beta2-receptors which are chiefly involved in broncho and vasodilation. It has no membrane-stabilizing effect nor partial agonist (intrinsic sympathomimetic) activity.
The stimulant effect of catecholamines on the heart is reduced or inhibited by S(-)Metoprolol. This leads to a decrease in heart rate, cardiac contractility, and cardiac output.
5.2 Pharmacodynamic properties
S (-) Metoprolol is a selective β1 adrenergic receptor blocker. It has a preferential effect on β1 receptors, chiefly located in the cardiac muscle. This preferential effect is however not absolute, and at higher doses, S (-) Metoprolol also inhibits β2 receptors which are abundantly present in the bronchial and vascular musculature. S (-) Metoprolol has no intrinsic sympathomimetic activity and membrane-stabilizing activity is detectable only at plasma concentrations much greater than required for β-blockade.
The antihypertensive effects of β blocking agents may be attributed to
i) Competitive antagonism of catecholamines at peripheral (cardiac) adrenergic neuron sites, leading to decreased cardiac output; ii) A central effect leading to reduced sympathetic outflow to the periphery; and iii) Suppression of renin activity.
S (-) Metoprolol has been shown to be an effective antihypertensive agent when used alone or as concomitant therapy with thiazide-type diuretics, at doses of 100-400 mg daily.
In addition, S (-) Metoprolol reduces the oxygen requirements of the heart at any given level of effort by blocking catecholamine-induced increases in heart rate, velocity and extent of myocardial contraction, and in blood pressure, thus making it useful in the long-term management of angina pectoris.
S (-) Metoprolol administered two or four times daily, has been shown to be an effective anti-anginal agent, reducing the number of angina attacks and increasing exercise tolerance.
5.3 Pharmacokinetic properties
Absorption
S (-) Metoprolol is well absorbed after oral administration, peak plasma concentrations occurring 1.5 - 2 hours after dosing. The bioavailability of a single dose is approximately 50%, increasing to approximately 70% during repeated administration. The bioavailability also increases if S (-) Metoprolol is given with food.
Distribution
Approximately 10% of S (-) Metoprolol in plasma is protein bound. S (-) Metoprolol crosses the placenta and is found in breast milk.
S (-) Metoprolol is extensively metabolized by enzymes of the cytochrome P450 system in the liver. The oxidative metabolism of S (-) Metoprolol is under genetic control with a major contribution of the polymorphic cytochrome P450 isoform 2D6 (CYP2D6). There are marked ethnic differences in the prevalence of the poor metabolizers (PM) phenotype. Approximately 7% of Caucasians and less than 1% of Orientals are PMs.
CYP2D6 poor metabolizers exhibit several-fold higher plasma concentrations of S (-) Metoprolol than extensive metabolizers with normal CYP2D6 activity. None of the metabolites of S (-) Metoprolol contribute significantly to its beta-blocking effect.
Elimination
Elimination is mainly by hepatic metabolism and the average elimination half-life is 3.5 hours (range 1 to 9 hours). Rates of metabolism vary between individuals, with poor metabolizers (approximately 10%) showing higher plasma concentrations and slower elimination than extensive metabolizers. Within individuals, however, plasma concentrations are stable and reproducible.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in rats at three oral dosage levels of up to 800 mg/kg/day (41 times, on a mg/m2 basis, the daily dose of 200 mg for a 60 kg patient), there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug-related were an increased incidence of generally mild focal accumulation of foamy macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg/day (18 times, on a mg/m2 basis, the daily dose of 200 mg for a 60-kg patient), benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, nor in the overall incidence of tumors or malignant tumors. This 21month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor. All genotoxicity tests performed on metoprolol tartrate (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonella/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) and metoprolol succinate (a Salmonella/mammalian-microsome mutagenicity test) were negative. No evidence of impaired fertility due to metoprolol tartrate was observed in a study performed in rats at doses up to 22 times, on a mg/m2 basis, the daily dose of 200 mg in a 60-kg patient.
7.0 Description
S(-)Metoprolol, is a beta 1-selective (cardioselective) adrenoceptor-blocking agent, for oral administration, available as prolonged-release tablets. Metoprolol succinate prolonged-release tablet has been formulated to provide a controlled and predictable release of S(-)Metoprolol for once-daily administration.
The tablets contain 11.875, 23.75, and 47.5 mg of S(-)Metoprolol succinate equivalent to 12.5, 25, and 50 mg of S(-)Metoprolol tartrate respectively. Its chemical name is (2S)-1-[4-(2-methoxyethyl)phenoxy]-3-(propan-2-ylamino)propan-2-ol. Its structural formula is: C15H25NO3
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf-life
24 Months
8.3 Packaging information
Strip of 10 tablets
8.4 Storage and handing instructions
The medical product does not require any special storage conditions
9.0 Patient counselling information
Heart failure patients should be advised if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
Advise patients if a dose is missed, the patient should take only the next scheduled dose (without doubling it). Patients should not interrupt or discontinue metoprolol succinate extended-release capsules without consulting the physician.
Advise patients
(1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with metoprolol succinate extended-release capsules has been determined;
(2) to contact the physician if any difficulty in breathing occurs;
(3) to inform the physician or dentist before any type of surgery that he or she is taking metoprolol succinate extended-release capsules.
Advise patients who are breastfeeding to monitor the infant for bradycardia, dry mouth, skin or eyes, and diarrhea or constipation.
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you:
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What KIMET XL® is and what it is used for
- What you need to know before you take KIMET XL®
- How to take KIMET XL®
- Possible side effects
- How to store KIMET XL®
- Contents of the pack and other information
1. What KIMET XL® is and what it is used for
KIMET XL® tablets contain S(-)Metoprolol.
S(-)Metoprolol succinate tablets contain S(-)Metoprolol tartrate and is one of a group of medicines called beta-blockers. Beta-blockers slow the heartbeat, lessen the force with which the heart muscle contracts and reduce blood vessel contraction in the heart, brain, and throughout the body.
S(-)Metoprolol tablets are used to treat a number of different conditions including:
- High blood pressure
- Angina (chest pain)
- Some heart disorders, for example, heart attack or irregular heartbeats.
- They can also be used as part of the treatment for an overactive thyroid gland.
- S(-)Metoprolol tablets can be taken to help prevent migraine attacks.
2. What you need to know before you take KIMET XL®
Do not take KIMET XL®:
Do not take S(-)Metoprolol tablets if:
you are allergic to S(-)Metoprolol, any other beta-blocker drugs or any of the other ingredients of this medicine. If you think you may be allergic, talk to your doctor before taking this medicine.
- you are allergic to any other beta-blocker drugs,
- you have severe asthma or severe attacks of wheezing,
- you have certain serious heart or blood vessel disorders that shouldn’t be treated with beta-blockers (your doctor should be aware of these),
- you have low blood pressure,
- you have been told that you have high blood pressure due to a tumor near your kidney (phaeochromocytoma),
- you have been told that your blood is more acidic than normal (a condition called metabolic acidosis).
If any of the above applies to you, do not take KIMET XL® and talk to your doctor.
Warnings and precautions
Talk to your doctor before taking KIMET XL® Tablets if you:
- suffer from asthma, bronchitis or any similar lung disorder
- have problems with your heart (such as slow heart rate) or circulation (taking this medicine may make these worse)
- have diabetes
- suffer from any serious liver disease
- have had a severe allergic reaction to anything
- suffer from a rare form of angina called Prinzmetal's angina
- will be having an operation that requires a general anesthetic
- have psoriasis
If any of these apply to you, discuss your treatment with your doctor or pharmacist because this medicine might not be the right medicine for you.
If you are going to have a general anesthetic, tell the doctor or dentist in charge that you are taking S(-)Metoprolol tablets. If you are diabetic, take particular care with your blood sugar control since S(-)Metoprolol tablets may make you less aware of low blood sugar levels.
The doctor will want to keep an eye on your heart and thyroid function while you are taking this medicine. You might also need regular eye examinations
Children and adolescents
S(-)Metoprolol tablets are not recommended for the treatment of children or adolescents.
Other medicines and KIMET XL® Tablets
Tell your doctor or pharmacist if you are taking, have taken or might take any other medicines. This includes medicines you can buy without a prescription, including herbal medicines. This is because S(-)Metoprolol can affect the way some medicines work. Also, some medicines can affect the way S(-)Metoprolol works.
In particular, tell your doctor or pharmacist if you are taking any of the following:
- Medicines for high blood pressure (including prazosin, clonidine and drugs called calcium channel blockers such as verapamil or diltiazem)
- Other beta-blockers (including those used in the form of eye drops)
- Drugs which affect the peripheral circulation (fingers and toes) such as ergotamine which can be used to treat migraine
- Medicines to treat depression
- Medicines to treat other mental illnesses
- Antiretroviral drugs used to treat AIDS and some other conditions
- Antihistamines (including medicines that you can buy without a prescription for hay fever and other allergies, colds and other conditions)
- Drugs to prevent malaria
- Medicines to treat fungal infections
- Medicines which affect liver enzymes, such as cimetidine used to treat stomach ulcers and rifampicin used to treat tuberculosis
- Medicines for heart problems including angina, such as amiodarone, digoxin, nitrates and anti-arrhythmic drugs
- Insulin and other drugs to treat diabetes
- Drugs called NSAIDs used to treat pain and inflammation
- A local anaesthetic called lidocaine.
S(-)Metoprolol tablets with alcohol
Be careful when drinking alcohol - it may affect you more than usual.
Pregnancy and breast-feeding
S(-)Metoprolol is not recommended during pregnancy or breastfeeding. If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
If you feel dizzy or sleepy, or if you have problems with your eyes when you start to take these tablets, do not drive or use machinery until these effects have worn off.
3. How to take KIMET XL® Tablets
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The doctor will tell you how many S(-)Metoprolol tablets to take and when to take them. The dose you are prescribed will depend on the condition you have and how severe it is. The dose will be on the pharmacist’s label. Check the label carefully. If you are not sure, ask your doctor or pharmacist. Keep taking your tablets for as long as you have been told unless you have any problems. In that case, check with your doctor. Swallow your tablets with a drink of water.
The recommended doses are:
High blood pressure: The usual starting dose is 12.5 to 50 mg a day. This can be increased by your doctor if necessary.
Angina (Chest pain): The usual dose is 25-50 mg taken two or three times a day.
For other conditions, the usual total daily dose is between 50 and 100 mg. Your doctor will choose a suitable starting dose and monitor your progress. Your doctor may also recommend that you take a lower dose of 12.5 mg based on your condition.
If you take more KIMET XL® than you should
If you have taken too many tablets of KIMET XL®, tell your doctor at once or contact your nearest hospital casualty department. Take your medicine with you so that people can see what you have taken.
If you forget to take or give KIMET XL®
If you forget to take a dose, take it when you remember, and then take your next dose at the usual time. Do not take a double dose to make up for a forgotten tablet.
If you stop taking KIMET XL®
Do not stop taking your tablets suddenly as this may cause your condition to get worse. Ask your doctor first. When discontinuing the treatment, the dose should be withdrawn gradually over a period of 10 days, the doses diminishing to 12.5 mg for the last 6 days.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects listed below have been reported. Common: may affect up to 1 in 10 people
- Headache, dizziness, or unusual tiredness.
- Slow heartbeat.
- Low blood pressure which might make you faint or dizzy.
- Feeling short of breath when exercising.
- Feeling or being sick, stomach ache.
Rare: may affect up to 1 in 1,000 people
- Sleep disorders such as sleepiness, sleeplessness or nightmares.
- Feeling less alert.
- Coldness, numbness or tingling in your hands and feet.
- Depression.
- Muscle cramps Heart failure or irregular heartbeat.
- Water retention (oedema).
- Breathlessness or wheeziness (bronchospasm).
- Diarrhoea or constipation.
- Skin rash and/or itching.
Very rare: may affect up to 1 in 10,000 people
- Weight gain.
- Hallucinations or personality disorders.
- Dry or sore eyes or problems with vision.
- Tinnitus or hearing problems.
- Gangrene.
- Runny nose, dry mouth.
- Changes in the results of liver function tests.
- Bruising or increased sensitivity of the skin to sunlight, worsening of psoriasis. Increased sweating, loss of hair. Impotence or loss of libido.
- Painful joints.
- Chest pain.
The following have also been reported:
- Confusion
- Abnormal levels of certain types of fats such as cholesterol or triglycerides in the blood.
- Abnormal curvature of the penis with painful erections (known as Peyronie’s disease)
- Retroperitoneal fibrosis where abnormal scar tissue occurs behind the membrane that lines the cavity of the abdomen.
- This may present with pain in the back, groin or the lower abdomen.
- Hepatitis
Do not be alarmed by this list - most people take S(-)Metoprolol without any problems. If any of the symptoms become troublesome, or if you notice anything else not mentioned here, please go and see your doctor as they may want to give you a different medicine.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store KIMET XL®
- Keep out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label and carton.
- The expiry date refers to the last day of that month. This medicinal product does not require any special storage conditions.
- Do not throw away any medicines via wastewater or household waste.
- Ask your pharmacist how to throw away medicines you no longer use.
- These measures will help protect the environment.
6. Contents of the pack and other information
What KIMET XL® tablets contain
KIMET XL®-12.5
Each tablet contains S(-)Metoprolol Succinate 11.875 mg equivalent to S(-)Metoprolol
Tartrate 12.50 mg.
KIMET XL®-25
Each tablet contains S(-)Metoprolol Succinate 23.75 mg equivalent to S(-)Metoprolol
Tartrate 25 mg.
KIMET XL®-50
Each tablet contains S(-)Metoprolol Succinate 47.50 mg equivalent to S(-)Metoprolol
Tartrate 50 mg.
KIMET XL®-50
Each tablet contains S(-)Metoprolol Succinate 47.50 mg equivalent to S(-)Metoprolol
Tartrate 50 mg.
What KIMET XL® looks like and contents of the pack
KIMET XL® is available in packs of 10 tablets.