Lafaxid-10 tablets
Therapy Area
Gastrointestinal
1.0 Generic name
Lafutidine Tablets
(brand name- LAFAXID-10 tablet)
2.0 Qualitative and quantitative composition
Each film-coated tablet contains:
Lafutidine 10 mg.
Excipients q. s
Colour: Titanium Dioxide I P
3.0 Dosage form and strength
Film coated tablets
Lafutidine 10 mg
4.0 Clinical particulars
4.1 Therapeutic Indication
For the treatment of:
- gastric ulcers, duodenal ulcers and stromal ulcers.
- gastric mucosal lesions (erosion, hemorrhage, redness or edema) associated with acute gastritis and acute exacerbation of chronic gastritis.
4.2 Posology And Method of Administration
- Gastric ulcers, duodenal ulcers and stromal ulcers For Adults- 1 tablet two times daily, one after breakfast and one after evening meal or before sleeping. Dose must be adjusted according to patients age and symptoms.
- Gastric mucosal lesions (erosion, hemorrhage, redness or edema) associated with acute gastritis and acute exacerbation of chronic gastritis. For Adults-1 tablet once daily, after evening meal or before sleeping. Dose must be adjusted according to patients age and symptoms.
4.3 Contraindications
Patients with a history of drug hypersensitivity to any of the ingredients in the product.
4.4 Warning and Precautions
Lafutidine should be administered with care in patients with a history of drug hypersensitivity, impaired hepatic function, impaired renal function and patients on dialysis.
Use in the Elderly
Normally, because physiological function is deteriorated in the elderly, it is advisable to take such measures as a reduction in the dose and a prolongation of the administration interval under careful supervision.
4.5 Drug Interactions
Not known
4.6 Special populations
Pregnancy and Lactation
The safety of Lafutidine in pregnant women has not been established. Therefore, the administration of Lafutidine to pregnant women or women who may possibly be pregnant should be strictly limited to occasions where the therapeutic benefits outweigh the possible risks associated with the treatment. Animal studies (in rats) have shown that lafutidine is excreted in breast milk. Therefore, the mothers should be advised to discontinue breast feeding during treatment.
Pediatric use
This lafutidine drug can be used in children
4.7 Effects on ability to drive and use machines
The effects of the medicinal product on the ability to drive and use machines have not been studied.
4.8 Undesirable Effects
Constipation, diarrhea, drug rash, nausea or vomiting and dizziness are the adverse reactions observed during clinical trials. Other adverse effects of unknown frequency include abnormal changes in laboratory tests, hypersensitivity reactions (itching, redness of the skin, skin rash)
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via
email to: medico@zuventus.com
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9: Overdose
No data on overdosage with Lafutidine tablet is available.
5.0 Pharmacological properties
5.1 Mechanism of Action /5.2 Pharmacodynamic Properties
Lafutidine inhibits acid secretion via histamine H2-receptor blockade. It also possesses a cytoprotective efficacy, which comprises mucin biosynthesis, stimulation of gastric blood flow and promotion of epithelial restitution of damaged mucosa, mediated through capsaicin-sensitive sensory neurons and endogenous calcitonin gene-related peptide (CGRP). It increases the endothelial production of prostacyclin, which inhibits neutrophil activation and reduces inflammation. It also affects gastrointestinal functions by regulating gastrointestinal motility closely relate to changes in the plasma levels of somatostatin, CGRP and secretin.
5.3 Pharmacokinetic properties
When 10mg of lafutidine is orally administered to normal adult males in fasting conditions, peak plasma concentration of 265.15±49.84 ng/ml is achieved at 0.95±0.24 hours. Lafutidine is metabolized primarily in the liver. In vitro studies showed that lafutidine is mainly metabolized by CYP3A4 and partially metabolized by CYP2D6. Hydroxylated and sulfonyl lafutidine are the major metabolites. Most of the drug is excreted in bile while approximately 20% of the given dosage is excreted in urine. The plasma half-life is 1.92 ± 0.94 hours. Among the elderly, there was no difference in pharmacokinetics parameters between those with normal renal function and those with deteriorating renal function. In dialytic patients when administered without hemodialysis, the Cmax, AUC and the T1/2 values were 336±40 ng/ml, 2278±306 ng・hr/mL and 6.71±0.30 hours respectively while those after hemodialysis were 226±36 ng/ml, 853±128 ng・hr/mL and 4.57±0.2 hours respectively.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
No known animal toxicology data
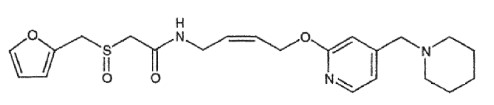
7.0 Description
Lafutidine is a second-generation histamine receptor antagonist (H2-RA) possessing an antisecretory effect as well as gastro-protective activity against several necrotizing agents independent of its antisecretory action.
Molecular Formula: C22H29N3O4S
Chemical name: 2-(Furfurylsulfinyl)-N-[4-[4-(piperidinomethyl)-2-pyridyl]oxy-(Z)-2-butenyl]acetamide Molecular weight: 431.56 Structural formula:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf Life
Refer on the pack.
8.3 Packaging Information
3 composite blister strips of 3 x 10 tablets each.
8.4 Storage and Handling Instructions
Store in a cool dry place.
Keep out of reach of children.
9.0 Patient counseling information
- Take Lafutidine before going to bed if you are taking this medicine once a day. It is very effective in controlling stomach acid that is released when you are asleep.
- If you are also taking other medications to treat acidity (e.g., antacid), take them 2 hours before or after taking Lafutidine.
- Avoid taking soft drinks, citrus fruits like orange and lemon, which can irritate the stomach and increase acid secretion.
- Inform your doctor if you do not feel better after taking Lafutidine for 2 weeks as you may be suffering from some other problems.
- Inform your doctor if you have ever been diagnosed with kidney disease as dose of your medicine may need to be adjusted.
- Do not stop taking the medication without talking to your doctor.
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What Lafaxid-10 is and what it is used for
- What you need to know before you take Lafaxid-10
- How to take Lafaxid-10
- Possible side effects
- How to store Lafaxid-10
- Contents of the pack and other information
1. What Lafaxid-10 is and what it is used for
Lafaxid-10 contains Lafutidine which belongs to a group of medicines called H2−receptor antagonists. These work by reducing the amount of acid you produce in your stomach. It can be used to relieve the symptoms of indigestion and heartburn caused by too much acid in the stomach.
Lafutidine is used to treat the following:
- Ulcer is in your duodenum, stomach.
- Gastroesophageal reflux disease (GERD) occurs when stomach acid repeatedly flows back into the tube connecting your mouth and stomach (esophagus)
- Gastric mucosal lesions (erosion, hemorrhage, redness or edema) associated with gastritis
2. What you need to know before you take Lafaxid-10
Do not take Lafutidine if:
- You are allergic to Lafutidine, other H2−receptor antagonists or any of the other ingredients of this medicine
- If you are under 6 years’ old
- If you are pregnant, breastfeeding, or trying to become pregnant, unless your doctor tells you to.
Warnings and precautions
Talk to your pharmacist or doctor:
- If you have any of the following symptoms – these may be a sign of a more serious problem that needs different treatment
- You have an ulcer in your stomach or intestine, or you think you may have one
- You are middle aged or older and this is the first time you have had these symptoms, or
- your symptoms have recently changed
- You have lost weight without trying, difficulty in swallowing, stomach pain
- If you have liver or kidney problems
- If you have regular medical appointments for any reason
- If you have any other illness, or take any medicines on a regular basis (including medicines prescribed by your doctor, and any you have bought for yourself)
- If you are taking any non-steroidal anti-inflammatory drugs, e.g. ibuprofen or aspirin, especially if you are elderly
- If you have a condition called porphyria (a rare blood disease) or you have ever had this condition
- There is a possibility of a malignant growth (tumour) being present in your stomach
- You have been taking a high dose of Lafutidine for a long time. Your doctor may monitor your blood count and liver function
Other important information
Information about some of the ingredients in this medicine: Castor oil may cause a
stomach upset or diarrhoea.
Do not drink alcohol (wine, beer, spirits) whilst taking this medicine.
If you take other medicines
Before you take these tablets, make sure that you tell your pharmacist about ANY other
medicines you might be using at the same time, particularly the following:
- Non-steroidal anti-inflammatory drugs
- Antacids (for indigestion)
- Sucralfate (for ulcers)
- Procainamide (for abnormal heartbeats)
- Theophylline (for asthma)
- Ketoconazole (for fungal infections)
- Glipizide (for diabetes)
If you take other antacids for indigestion, or sucralfate to treat ulcers and your doctor tells you that you can take this medicine as well, you must separate the times when you take them. You must take these tablets at least two hours before the antacid or sucralfate. If you are unsure about interactions with any other medicines, talk to your pharmacist. This includes medicines prescribed by your doctor and medicine you have bought for yourself, including herbal and homeopathic remedies.
Pregnancy and breast−feeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
This product should only be used during pregnancy and breast-feeding only considered essential.
Driving and using machines
These tablets have no known effects on ability to drive or operate machinery.
3. How to take Lafaxid
Always take Lafutidine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
- The recommended dose is one tablet once or twice daily according to age and severity of disease symptoms.
- Swallow one tablet with glass of water as soon as you have symptoms. If symptoms persist or recur take another tablet. Do not take more than 2 tablets in one day.
- The score line is only there to help you break the tablet if you have difficulty swallowing it whole.
- Lafutidine can be taken with or without food.
Patients with Kidney disorders/on dialysis
- If you suffer from kidney disorders, your doctor is likely to reduce your dose.
- Lafutidine should be administered at the end of dialysis or after since some of the active ingredient is removed by dialysis.
Use in children
Lafutidine can be used in children.
If you take more Lafutidine than you should
If you accidentally take too many tablets, contact your doctor or nearest hospital emergency department immediately for advice. Remember to take this leaflet or any remaining tablets with you.
If you forget to take your Lafutidine
Take it as soon as you remember, unless it is nearly time for your next dose. If you miss a dose, do not take a double dose to make up for a forgotten dose.
If you stop taking Lafutidine
It is important that you keep taking Lafutidine for as long as your doctor has told you to. In case of long-standing ulcer disease, abrupt withdrawal after symptom relief should be avoided. If you have any further questions on the use of this medicine, ask your doctor or pharmacist
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects are mentioned below:
Constipation, diarrhea, drug rash, nausea or vomiting and dizziness are the adverse reactions observed during clinical trials.
Other adverse effects of unknown frequency include abnormal changes in laboratory tests, hypersensitivity reactions (itching, redness of the skin, skin rash)
! If you get any of these serious side effects, stop taking the tablets. See a doctor at once:
- Difficulty in breathing, swelling of the face, neck, tongue or throat, chest pain, unexplained
- fever, fainting (severe allergic reactions)
- Fast, slow or irregular heartbeat
- Severe stomach pain (this may be caused by a swollen pancreas)
These effects are rare and happen to one person in 1000 to 10,000 who take this medicine
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Lafaxid
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of that month. This medicine does not require any special temperature storage conditions. Store in the original package in order to protect it from moisture. Do not use this medicine if you notice that the pack is damaged or shows signs of tampering. Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Lafaxid contains
The active substances are lafutidine.
Each film-coated tablet contains:
Lafutidine 10 mg.
Excipients q. s
Color: Titanium Dioxide I P
Pack size:
3 composite blister strips of 3 × 10 tablets each.