Lornit Infusion
Therapy Area
Gastrointestinal
1.0 Generic name
L-Ornithine L -Aspartate Infusion Concentrate
2.0 Qualitative and quantitative composition
Each ml contains:
L-Ornithine L-Aspartate 0.5 gm
Water for Injections IP q.s.
3.0 Dosage form and strength
Concentrate for solution for infusion.
One ampoule of 10 ml contains 5 g L-Ornithine-L-Aspartate.
4.0 Clinical particulars
4.1 Therapeutic indications
- Latent and manifest hepatic encephalopathy.
- For pre coma & coma due to conditions (like cirrhosis) leading to impaired hepatic detoxification.
4.2 Posology and method of administration
The recommended dose is up to 4 ampoules (20 gram) per day. In case of pre-coma or coma up to 8 ampoules (40 gram) within 24 hours, depending on the severity of the condition.
The ampoules are added to an infusion solution before use, and infused in this form:
- Maximum Infusion Rate: 5 grams of L-ornithine L-aspartate (corresponding to the content of 1 ampoule) per hour.
- Maximum concentration: 6 ampoules per 500 ml of infusion solution.
- Infusion solutions: Normal saline, Dextrose, Ringer Lactate etc.
Lornit infusion concentrate must not be administered into an artery. Experience in children is limited.
4.3 Contraindications
- Hypersensitivity to L-ornithine L-aspartate.
- Severely impaired renal function (renal insufficiency- A serum creatinine > 3 mg/100 ml can be used as a guidance value.
4.4 Special warnings and precautions for use
At high doses of Lornit infusion concentrate, serum and urine urea levels should be monitored.
If liver function is substantially impaired, the infusion rate must be adjusted to the individual patient in order to prevent nausea and vomiting.
No data are so far available on the use of the drug in children.
4.5 Interaction with other medicinal products and other forms of interaction No interaction studies have been performed. Up to now interactions are not known.
4.6 Pregnancy and lactation
There are no clinical data available on the use of Lornit infusion concentrate in pregnancy.
L-ornithine L-aspartate has been investigated for reproduction toxicity only to a limited extent in experimental animal studies. The administration of Lornit infusion concentrate in pregnancy should therefore be avoided. If treatment with Lornit is nevertheless thought to be necessary, the benefits and risks should be carefully assessed.
It is not known whether L-ornithine L-aspartate passes into breast milk. Administration of Lornit should therefore be avoided during lactation. If treatment with Lornit is nevertheless thought to be necessary, the benefits and risks should be carefully assessed.
4.7 Effects on ability to drive and use machines
Depending on the underlying disease, the ability to drive and operate machines may also be impaired on treatment with L-ornithine L-aspartate.
4.8 Undesirable effects
| Very common: | (>1/10) |
| Common: | (>1/100, <1/10) |
| Uncommon: | (>1/1000, <1/100) |
| Rare: | (>1/10000, <1/1000) |
| Very rare: | (<1/10000), not known (cannot be estimated from the available data) |
Gastrointestinal disorders
Uncommon: Nausea
Rare: Vomiting
Generally, however, these symptoms are transient, and do not necessitate discontinuation of treatment with this medicinal product. They disappear on reduction of the dose or infusion rate.
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
So far signs of intoxication have not been observed following an overdose of L-ornithine L-aspartate. Cases of overdose require symptomatic treatment.
5.0 Pharmacological properties
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Liver therapy, ATC code: A05BA
In human body, L-ornithine L-aspartate acts on two key ammonia detoxification pathways – urea synthesis and glutamine synthesis – via the amino acids ornithine and aspartate. Urea synthesis takes place in the periportal hepatocytes, in which ornithine serves both as an activator of the two enzymes ornithine carbamoyl
transferase and carbamoyl phosphate synthetase and as a substrate for urea synthesis. Glutamine synthesis is localised in the perivenous hepatocytes. Under pathological conditions in particular, aspartate and other dicarboxylates – including metabolic products of ornithine – are taken up into the cells where they are used in the form of glutamine to bind ammonia.
Both physiologically and pathophysiologically glutamate serves as an ammonia-binding amino acid. The resulting amino acid glutamine not only provides a non-toxic form for the excretion of ammonia but also activates the important urea cycle (intercellular glutamine exchange).
Under physiological conditions ornithine and aspartate are not limiting for urea synthesis. Experimental studies in animal’s point to increased glutamine synthesis as a mechanism of the ammonia- lowering effect. Some clinical studies have shown an improvement in the ratio of branched chain to aromatic amino acids.
5.2 Pharmacokinetic properties
Ornithine and aspartate have a short elimination half-life of 0.3–0.4 hours. Some of the aspartate is excreted unchanged in the urine.
5.3 Preclinical safety data
Based on pharmacological safety studies, preclinical data show that with correct use there is no particular risk of toxicity following repeated administration or mutagenicity in humans. No studies on carcinogenic potential have been carried out. In a dose discovery study, L-ornithine L-aspartate was investigated for reproduction toxicity only to a limited extent.
6.0 Nonclinical properties
None stated
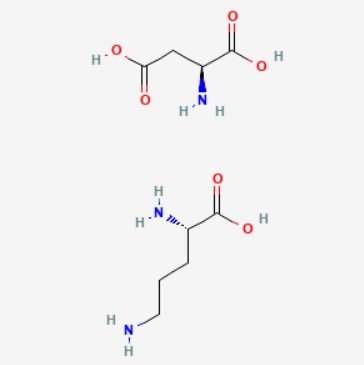
7.0 Description
L-Ornithine-L-Aspartate is a stable salt of the amino acids ornithine and aspartic acid. Reduces blood ammonia concentrations and improves mental function when administered IV in patients with hepatic encephalopathy.
Molecular Formula: C9H19N3O6
Molecular Weight: 265.26 g/mol
8.0 Pharmaceutical particulars
8.1 List of excipients
Water for injections
8.2 Incompatibilities
As no compatibility studies have been performed the medicinal product must not be mixed with other medicinal products.
8.3 Shelf life
Refer on pack
8.4 Special precautions for storage
Do not store above 25ºC.
Store in a cool & dark place.
Keep out of reach of children.
8.5 Packaging information
The concentrate to be made up into solution for infusion is presented in amber-coloured glass ampoules. An ampoule of 10 ml.
8.6 Special precautions for disposal
No special requirements.
About leaflet
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- Do not pass this medicine on to others. It may harm them, even if their symptoms are the same as yours. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
- What Lornit infusion concentrate is and what it is used for
- Before you use Lornit infusion concentrate
- How to use Lornit infusion concentrate
- Possible side effects
- How to store Lornit infusion concentrate
- Further information
1. What Lornit Infusion Concentrate is and What It is Used for
Lornit infusion concentrate is a medicine which stimulates ammonia detoxification by increasing urea synthesis in the liver. Furthermore, it serves ammonia detoxification in the tissues outside the liver.
Lornit infusion concentrate is used in the treatment of depression of consciousness due to liver failure (latent and manifest hepatic encephalopathy).
2. Before You Use Lornit Infusion Concentrate
Do not use Lornit infusion concentrate
- if you react hypersensitively to L-Ornithine-L-Aspartate
- if you suffer from severely impaired kidney function (renal insufficiency). A serum creatinine value over 3 mg / 100 ml can be used as a guideline value.
Take special care with Lornit infusion concentrate
- if high doses of Lornit infusion concentrate are used. The urea level in the serum and urine should be monitored by your doctor.
- if liver function is severely impaired. In order to avoid nausea and vomiting, your doctor will adjust the infusion rate on an individual basis.
No data are so far available on the use of the drug in children.
Taking other medicines:
Please tell your doctor or pharmacist if you are taking/using or have recently taken/used any other medicines, including medicines obtained without a prescription.
To date, no interactions are known.
Pregnancy and breast-feeding
Safety of use of Lornit infusion concentrate has not been established during pregnancy and lactation. If you wish to become pregnant, are already pregnant or are breastfeeding, please inform your doctor.
Ask you doctor or pharmacist for advice before taking any medicine.
Driving and using machines:
As a result of your illness, your ability to drive and use machines may be impaired during treatment with Lornit infusion concentrate.
3. How to Use Lornit Infusion Concentrate
Unless otherwise prescribed by your doctor, the usual dose is up to 4 ampoules daily.
On initial depression of consciousness (precoma) and clouding of consciousness (coma), your doctor may administer up to 8 ampoules within 24 hours depending upon the severity of the condition.
Mode of administration:
Lornit infusion concentrate is a concentrate for solution for infusion. Prior to use, your doctor will add the ampoule contents to a solution for infusion and will infuse Lornit infusion concentrate in this form.
Lornit infusion concentrate can be easily mixed with the usual infusion solutions. On grounds of venous compatibility, however, your doctor should dissolve no more than 6 ampoules per 500 ml infusion.
The maximum infusion rate is 5 g L-ornithine-L-aspartate (corresponding to the contents of one ampoule) per hour.
Lornit infusion concentrate may not given as an intra-arterial administration.
If you have the impression that the effect of Lornit infusion concentrate is too strong or too weak, please talk to your doctor or pharmacist.
If you use more Lornit infusion concentrate than you should:
No signs of intoxication have been observed following an overdose of L-ornithine-L-aspartate so far. If necessary, your doctor will treat the symptoms.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, Lornit infusion concentrate can cause side effects, although not everybody gets them.
Possible side effects of Lornit infusion concentrate:
Uncommon: Nausea
Rare: Vomiting
These side effects are generally transient, however, and do not require discontinuation of the medicine. They resolve following a dose reduction or reduction of the infusion rate.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top of the home page.
- By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to Store Lornit Infusion Concentrate
Keep out of the reach and sight of children.
Do not use this medicine after the expiry date given on the outer carton/container.
Do not store above 25°C.
6. Further Information
What Lornit infusion concentrate contains
The active substance is L-ornithine-L-aspartate.
Each ml contains: L-Ornithine L-Aspartate 0.5 gm
1 ampoule with 10 ml contains 5 g L-ornithine-L-aspartate.
The other ingredient is water for injections.
What Lornit infusion concentrate looks like and contents of the pack
Lornit infusion concentrate is a clear solution.
An ampoule of 10 ml.