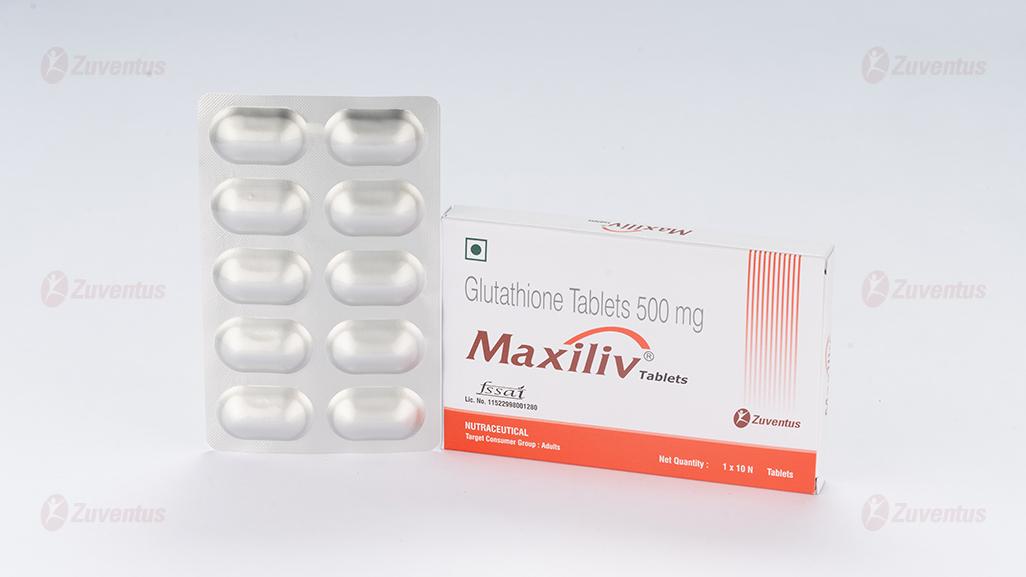
Maxiliv Tablet
Therapy Area
Gastrointestinal
1.0 Name of the medicinal product
Glutathione tablet
2.0 Qualitative and quantitative composition
Each film coated tablet contains
Glutathione……………………………………………500 mg
3.0 Dosage form and strength
Tablet 500 mg
4.0 Clinical particulars
4.1 Therapeutic indication
- Alcoholic fatty liver
- Alcoholic liver fibrosis
- Alcoholic liver cirrhosis
- Hepatitis
4.2 Posology and method of administration
Adults: One Maxiliv tablet (500 mg) once or twice daily.
4.3 Contraindications
Patients who show hypersensitivity to reduced glutathione.
4.4 Special warnings and precautions for use
- Administer Maxiliv tablet under the supervision of a doctor.
- Keep out of reach of children.
- If unusual symptoms such as eruption, pale face, blood pressure drop, or difficulty in breathing occur during therapy, discontinue the drug immediately.
4.5 Drugs interactions
Vitamin K, Vitamin B12, Calcium pantothenate and antihistamines can affect the bioavailability of glutathione.
4.6 Use in special populations
Pregnancy and Lactation
Maxiliv tablet should not be administered to a pregnant and lactating woman unless clinical benefit outweighs the risks.
Geriatric
Appropriate dose reduction is required during therapy.
4.7 Effects on ability to drive and use machines
It is not known whether Maxiliv Tablet alters the ability to drive. Do not drive if you experience any symptoms that affect your ability to concentrate and react.
4.8 Undesirable Effects
According to the scientific literature, there are no risks or side effects of oral administration glutathione. However, there have been anecdotal reports of transitory increase in flatulence, gastrointestinal irritation which diminishes after several days of consistent use.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
No specific antidote is available for the treatment of glutathione overdosage. Symptomatic treatment should be provided.
5.0 Pharmacological properties
5.1 Mechanism of Action/ Pharmacodynamic properties
Glutathione is an extremely important cell protectant which directly quenches reactive hydroxyl free radicals, other oxygen-centered free radicals, and radical centers on DNA and other biomolecules. It is an essential cofactor for many enzymes which require thiol-reducing equivalents, and helps to keep redox-sensitive active sites on enzymes in the necessary reduced state. Higher-order thiol cell systems like metallothioneins, thioredoxins, and other redox regulator proteins are ultimately regulated by reduced form of glutathione (GSH) levels and the ratio of GSH/GSSG (oxidized GSH).
GSH/GSSG balance is crucial for homeostasis, stabilizing the cellular biomolecular spectrum, and facilitating cellular performance and survival. GSH and its metabolites also interface with energetics and neurotransmitter syntheses, through several prominent metabolic pathways. GSH availability down-regulates the pro-inflammatory potential of leukotrienes and other eicosanoids. Recently discovered S-nitroso metabolites, generated in-vivo from GSH and NO (nitric oxide) further diversify GSH’s impact on metabolism.
5.2 Pharmacokinetic properties
A 6-month randomized, double-blinded, placebo-controlled trial of oral GSH (250 or 1,000 mg/day) on GSH levels in blood, erythrocytes, plasma, lymphocytes and exfoliated buccal mucosal cells was conducted in 54 non-smoking adults.
GSH levels in blood increased after 1, 3 and 6 months versus baseline at both doses. At 6 months, mean GSH levels increased 30-35 % in erythrocytes, plasma and lymphocytes and 260 % in buccal cells in the high-dose group (P < 0.05). GSH levels increased 17 and 29 % in blood and erythrocytes, respectively, in the low-dose group (P < 0.05). In most cases, the increases were dose and time dependent, and levels returned to baseline after a 1-month washout period. A reduction in oxidative stress in both GSH dose groups was indicated by decreases in the oxidized to reduced glutathione ratio in whole blood after 6 months.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
No known animal toxicology data
7.0 Description
Glutathione (GSH) is a physiological tripeptide- a chain of three amino acid- cysteine, glycine and glutamic acid. It is used for the detoxification of electrophilic metabolites. It is a very efficient free radical scavenger, preventing damage to important cellular components caused by reactive oxygen species such as free radicals and peroxides.
Chemical Name: (2S)-2-Amino-4-[1-(carboxymethyl)carbamoyl-(2R)-2- sulfanylethylcarbamoyl] butanoic acid.
Molecular Formula: C10H17N3O6S
Molecular Weight: 307.32 g/mol
8.0 Pharmaceutical particulars
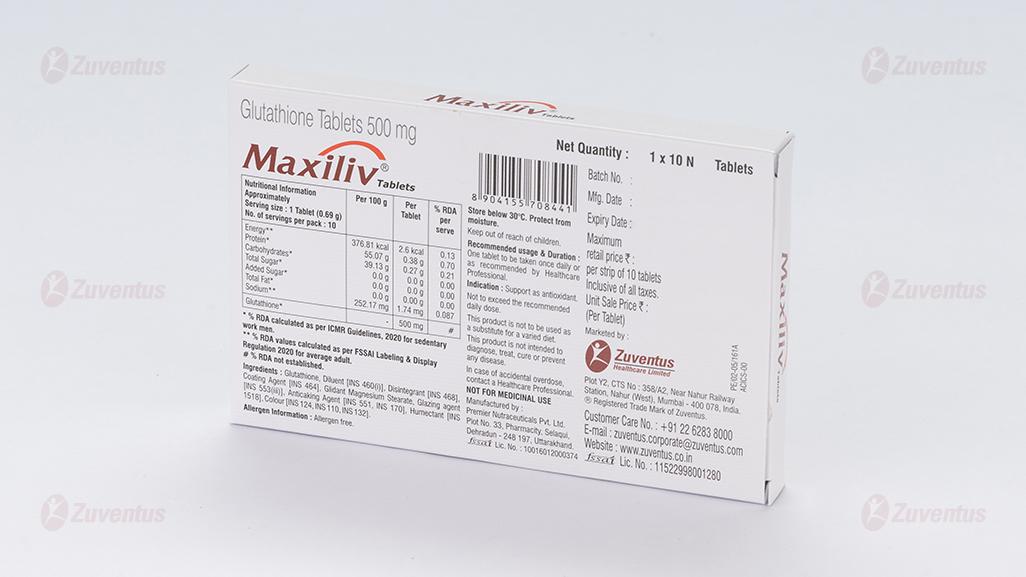
8.1 List of excipients
8.2 Incompatibilities
8.3 Shelf life
8.4 Special precautions for storage
9.0 Patient counselling information
- Inform your doctor if you have past or current history of asthma.
- Seek immediate medical attention if you have difficulty in breathing after taking the drug.
- Tell your doctor if you are or planning to become pregnant or are breastfeeding.
- Do not take if allergic to glutathione, milk protein or any of its ingredients.
- Do not take if patients had organ transplantation.
- Avoid if suffering from asthma.
- Do not take if patient is Pregnant and breastfeeding women.
12.0 Date of revision
28.11.2024
About leaflet
Please read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
- What Maxiliv Tablet is and what it is used for
- What you need to know before you take Maxiliv Tablet
- How to take Maxiliv Tablet
- Possible side effects
- How to store Maxiliv Tablet
- Contents of the pack and other information
1. What Maxiliv Tablet is and what it is used for
Maxiliv Tablet contains the active substance glutathione. It is used for the treatment of:
- Alcoholic fatty liver
- Alcoholic liver fibrosis
- Alcoholic liver cirrhosis
- Hepatitis
2. What you need to know before you take Maxiliv Tablet
Do not take Maxiliv Tablet if you:
- Are allergic to glutathione or any of the other ingredients of this medicine.
- Have had an organ transplantation.
- Are pregnant or breastfeeding.
Warnings and precautions:
- Administer Maxiliv Tablet under the supervision of a doctor.
- Keep out of reach of children.
- If you experience unusual symptoms such as rash, pale face, drop in blood pressure, or difficulty in breathing, stop taking the medicine and seek medical attention immediately.
Other medicines and Maxiliv Tablet:
- Vitamin K, Vitamin B12, Calcium pantothenate, and antihistamines can affect the bioavailability of glutathione.
Pregnancy and breastfeeding:
- Maxiliv Tablet should not be used during pregnancy and breastfeeding unless the potential benefit outweighs the risks.
Driving and using machines:
- It is not known whether Maxiliv Tablet affects your ability to drive. Do not drive if you experience symptoms that affect your ability to concentrate and react.
3. How to take Maxiliv Tablet
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Adults:
Take one Maxiliv Tablet (500 mg) once or twice daily.
If you use more Maxiliv Tablet than you should
Tell your doctor if you accidentally use more than you were told.
If you forget to use Maxiliv Tablet
If you forget to take at the right time, use it as soon as you remember, then carry on as before. Do not take a double dose to make up for a forgotten dose.
If you stop using Maxiliv Tablet
Do not stop your treatment even if you feel better unless told to do so by your doctor.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
There are no known risks or side effects of oral administration of glutathione. However, there have been anecdotal reports of transient increases in flatulence and gastrointestinal irritation, which diminish after several days of consistent use.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page. Website link: https://www.zuventus.com/drug-safety-reporting.
By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store Maxiliv Tablet
- Keep this medicine out of the sight and reach of children.
- Store in a cool, dry place away from direct sunlight.
- Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of that month.
- Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Maxiliv Tablet contains:
The active substance is glutathione (500 mg per tablet).
Revised on 11/24