Maxstine M Tablets
Therapy Area
Respiratory
1.0 Generic name
Montelukast & Bilastine Tablets
2.0 Qualitative and quantitative composition
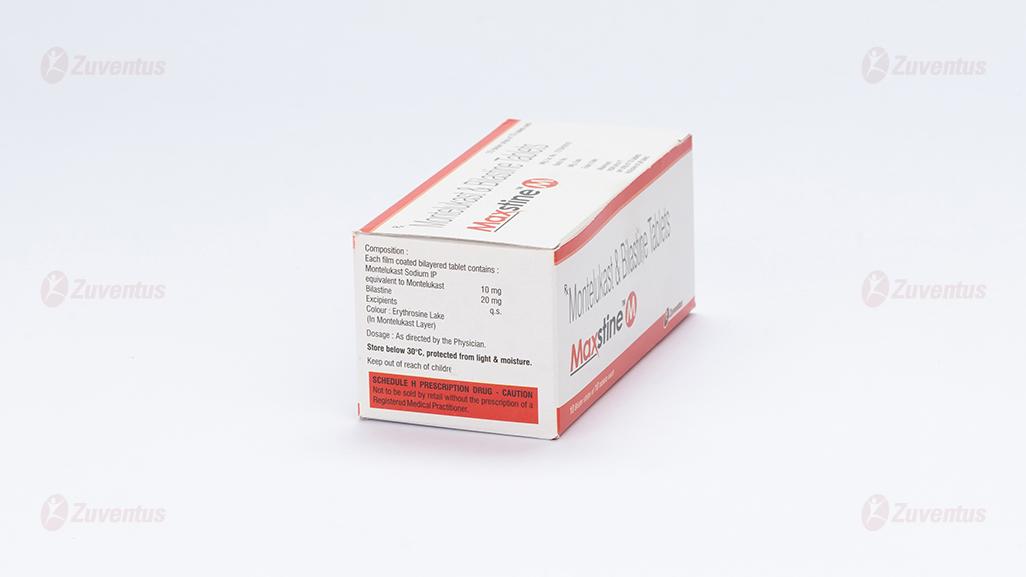
Each film coated bilayered tablet contains :
Montelukast Sodium IP equivalent to Montelukast 10 mg
Bilastine 20 mg
Excipients q.s.
Colour : Erythrosine Lake
(In Montelukast Layer)
3.0 Dosage form and strength
Tablet
4.0 Clinical particulars
4.1 Therapeutic indication
For treatment of allergic rhinitis in adults
4.2 Posology & method of administration
Adults & adolescents (15 years of age and over)
One Maxstine M tablet per day
Special populations
Elderly
No dosage adjustments are required in elderly patients.
Renal impairment
Studies conducted in adults in special risk groups (renally impaired patients) indicate that it is not necessary to adjust the dose of Maxstine M in adults.
Hepatic impairment
There is no clinical experience in adult patients with hepatic impairment. However, since Maxstine M is not metabolized and is eliminated as unchanged in urine and faeces, hepatic impairment is not expected to increase systemic exposure above the safety margin in adult patients. Therefore, no dosage adjustment is required in adult patients with hepatic impairment.
Method of administration
The tablet is to be swallowed with water. It is recommended to take the daily dose in one single intake. The tablet should be taken one hour before or two hours after intake of food or fruit juice
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients
4.4 Special warnings and precautions for use
• In patients with moderate or severe renal impairment coadministration of Bilastine with P-glycoprotein inhibitors, such as e.g, Ketoconazole, Erythromycin, Cyclosporine, Ritonavir or Diltiazem, may increase plasmatic levels of Maxstine M and therefore increase the risk of adverse effects of Bilastine. Therefore, coadministration of Bilastine and P-glycoprotein inhibitors should be avoided in patients with moderate or severe renal impairment.
• Patients should be advised never to use oral Montelukast to treat acute asthma attacks and to keep their usual appropriate rescue medication for this purpose readily available. If an acute attack occurs, a short-acting inhaled b-agonist should be used. Patients should seek their doctor's advice as soon as possible if they need more inhalations of short-acting b-agonists than usual.
• Montelukast should not be substituted abruptly for inhaled or oral corticosteroids.
• There are no data demonstrating that oral corticosteroids can be reduced when Montelukast is given concomitantly.
• In rare cases, patients on therapy with anti-asthma agents including Montelukast may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These cases have been sometimes associated with the reduction or withdrawal of oral corticosteroid therapy. Although a causal relationship with leukotriene receptor antagonism has not been established, physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. Patients who develop these symptoms should be reassessed and their treatment regimens evaluated.
• Treatment with Montelukast does not alter the need for patients with Aspirin-sensitive asthma to avoid taking Aspirin and other non-steroidal anti-inflammatory drugs.
• Neuropsychiatric events have been reported in adults, adolescents, and children taking Montelukast 10 mg film-coated tablets. Patients and physicians should be alert for neuropsychiatric events. Patients and/or caregivers should be instructed to notify their physician if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with Montelukast 10 mg film-coated tablets if such events occur
4.5 Drugs interactions
Bilastine
• Interaction with food
Food significantly reduces the oral bioavailability of Bilastine by 30%.
• Interaction with grapefruit juice
Concomitant intake of Bilastine 20 mg and grapefruit juice decreased Bilastine bioavailability by 30%. This effect may also apply to other fruit juices. The degree of bioavailability decrease may vary between producers and fruits. The mechanism for this interaction is an inhibition of OATP1A2, an uptake transporter for which Bilastine is a substrate. Medicinal products that are substrates or inhibitors of OATP1A2, such as Ritonavir or Rifampicin, may likewise have the potential to decrease plasma concentrations of Bilastine.
• Interaction with Ketoconazole or Erythromycin
Concomitant intake of Bilastine 20 mg o.d. and Ketoconazole 400 mg o.d. or Erythromycin 500 mg t.i.d. increased Bilastine AUC 2-fold and Cmax 2-3 fold. These changes can be explained by interaction with intestinal efflux transporters, since Bilastine is substrate for P-gp and not metabolised. These changes do not appear to affect the safety profile of Bilastine and Ketoconazole or Erythromycin, respectively. Other medicinal products that are substrates or inhibitors of P-gp, such as Cyclosporine, may likewise have the potential to increase plasma concentrations of Bilastine.
• Interaction with Diltiazem
Concomitant intake of Bilastine 20 mg o.d. and Diltiazem 60 mg o.d. increased Cmax of Bilastine by 50%. This effect can be explained by interaction with intestinal efflux transporters and does not appear to affect the safety profile of Bilastine.
• Interaction with Alcohol
The psychomotor performance after concomitant intake of Alcohol and 20 mg Bilastine o.d. was similar to that observed after intake of Alcohol and placebo.
• Interaction with Lorazepam
Concomitant intake of Bilastine 20 mg o.d. and Lorazepam 3 mg o.d. for 8 days did not potentiate the depressant CNS effects of Lorazepam.
Paediatric population
Interaction studies have only been performed in adults. As there is no clinical experience regarding the interaction of Bilastine with other medicinal products, food or fruit juices in children, the results obtained in adult interactions studies should be at present taken into consideration when prescribing Bilastine to children. There are no clinical data in children to state whether changes to the AUC or Cmax due to interactions affect the safety profile of Bilastine.
Montelukast
• Montelukast may be administered with other therapies routinely used in the prophylaxis and chronic treatment of asthma. In drug-interactions studies, the recommended clinical dose of Montelukast did not have clinically important effects on the pharmacokinetics of the following medicinal products : Theophylline, Prednisone, Prednisolone, Oral contraceptives (Ethinyl Estradiol / Norethindrone 35/1), Terfenadine, Digoxin and Warfarin.
• The area under the plasma concentration curve (AUC) for Montelukast was decreased approximately 40% in subjects with co-administration of Phenobarbital. Since Montelukast is metabolised by CYP 3A4, 2C8, and 2C9, caution should be exercised, particularly in children, when Montelukast is co-administered with inducers of CYP 3A4, 2C8, and 2C9, such as Phenytoin, Phenobarbital and Rifampicin.
• In vitro studies have shown that Montelukast is a potent inhibitor of CYP 2C8. However, data from a clinical drug-drug interaction study involving Montelukast and Rosiglitazone (a probe substrate representative of medicinal products primarily metabolized by CYP 2C8) demonstrated that Montelukast does not inhibit CYP 2C8 in vivo. Therefore, Montelukast is not anticipated to markedly alter the metabolism of medicinal products metabolised by this enzyme (e.g., Paclitaxel, Rosiglitazone, and Repaglinide)
• In vitro studies have shown that Montelukast is a substrate of CYP 2C8, and to a less significant extent, of 2C9, and 3A4. In a clinical drug-drug interaction study involving Montelukast and Gemfibrozil (an inhibitor of both CYP 2C8 and 2C9) Gemfibrozil increased the systemic exposure of Montelukast by 4.4-fold. No routine dosage adjustment of Montelukast is required upon co-administration with Gemfibrozil or other potent inhibitors of CYP 2C8, but the physician should be aware of the potential for an increase in adverse reactions.
• Based on in vitro data, clinically important drug interactions with less potent inhibitors of CYP 2C8 (e.g., Trimethoprim) are not anticipated. Co-administration of Montelukast with Itraconazole, a strong inhibitor of CYP 3A4, resulted in no significant increase in the systemic exposure of Montelukast.
4.6 Use in special populations
Pregnancy
There are no or limited amount of data from the use of Maxstine M in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity, parturition or postnatal development. As a precautionary measure, it is preferable to avoid the use of Maxstine during pregnancy. Maxstine M tablets may be used during pregnancy only if it is considered to be clearly essential.
Nursing mothers
The excretion of Maxstine M in milk has not been studied in humans. Available pharmacokinetic data in animals have shown excretion of Maxstine M in milk. A decision on whether to continue / discontinue breast-feeding or to discontinue / abstain from Maxstine therapy must be made taking into account the benefit of breast-feeding for the child and the benefit of Maxstine M therapy for the mother
Fertility
There are no or limited amount of clinical data. A study in rats did not indicate any negative effect on fertility
4.7 Effects on ability to drive and use machines
Patients should be advised not to drive or use machines until they have established their own response to Maxstine M.
4.8 Undesirable effects
The following adverse reactions have been reported during clinical trials and are ranked using the following frequency :
Infections and infestations : Upper respiratory infection, Oral herpes.
Blood and lymphatic system disorders : Increased bleeding tendency, Thrombocytopenia.
Immune system disorder : Hypersensitivity reactions including anaphylaxis, Hepatic eosinophilic infiltration.
Metabolism and nutrition disorders : Increased appetite.
Psychiatric disorders : Anxiety, Insomnia, Dream abnormalities including nightmares, Somnambulism, Agitation including aggressive behaviour or hostility, Depression, Psychomotor hyperactivity (including irritability, restlessness, tremor), Disturbance in attention, Memory impairment, Hallucinations, Disorientation, Suicidal thinking and behaviour (suicidality), Obsessive-compulsive symptoms, Dysphemia.
Nervous system disorders : Somnolence, Headache, Dizziness, Drowsiness paraesthesia/hypoesthesia, Seizure.
Ear and labyrinth disorders : Uncommon - Tinnitus, Vertigo.
Cardiac disorders : Right bundle branch block, Sinus arrhythmia, Electrocardiogram QT prolonged, Palpitations.
Respiratory, thoracic and mediastinal disorders : Dyspnoea, Nasal discomfort, Nasal dryness, Epistaxis, Churg-Strauss Syndrome (CSS), Pulmonary eosinophilia.
Gastrointestinal disorders : Nausea, Stomach discomfort, Abdominal pain, Diarrhoea, Dry mouth, Dyspepsia, Gastritis, Vomiting.
Hepatobiliary disorders : Elevated levels of serum transaminases (ALT, AST), Hepatitis (including Cholestatic, Hepatocellular and mixed-pattern liver injury).
Skin and subcutaneous tissue disorders : Pruritus, Rash, Bruising, Urticaria, Angioedema, Erythema nodosum, Erythema multiforme. Musculoskeletal and connective tissue disorders : Arthralgia, Myalgia including muscle cramps.
General disorders and administration site conditions : Fatigue, Thirst, Pyrexia, Asthenia, Malaise, Oedema.
Renal and urinary disorders : Enuresis in children.
Investigations : Increased gammaglutamyltransferase, Increased ALT and AST, Increased Blood creatinine, Increased Blood TG, Increased weight.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
4.9 Overdose
Information regarding acute overdose of Bilastine is retrieved from the experience of clinical trials conducted during the development and the post-marketing surveillance. In clinical trials, after administration of Bilastine at doses 10 to 11 times the therapeutic dose (220 mg as single dose; or 200 mg/day for 7 days) to 26 adult healthy volunteer's frequency of treatment emergent adverse events was two times higher than with placebo. The adverse reactions most frequently reported were dizziness, headache and nausea. No serious adverse events and no significant prolongation in the QTc interval were reported. The information collected in the post-marketing surveillance is consistent with that reported in clinical trials. Critical evaluation of Bilastine's multiple dose (100 mg x 4 days) effect on ventricular repolarization by a “thorough QT/QTc cross-over study” involving 30 healthy adult volunteers did not show significant QTc prolongation
In chronic asthma studies, Montelukast has been administered at doses up to 200 mg/day to adult patients for 22 weeks and in short term studies, up to 900 mg/day to patients for approximately one week without clinically important adverse experiences. There have been reports of acute overdose in post-marketing experience and clinical studies with Montelukast. These include reports in adults and children with a dose as high as 1000 mg (approximately 61 mg/kg in a 42-month old child). The clinical and laboratory findings observed were consistent with the safety profile in adults and paediatric patients. In the event of overdose symptomatic and supportive treatment is recommended.
5.0 Pharmacological properties
5.1 Mechanism of action
Bilastine
Bilastine is a non-sedating, long-acting histamine antagonist with selective peripheral H1 receptor antagonist affinity and no affinity for muscarinic receptors. Bilastine inhibited histamineinduced wheal and flare skin reactions for 24 hours following single doses.
Montelukast
The cysteinyl leukotrienes (LTC4, LTD4, LTE4) are potent inflammatory eicosanoids released from various cells including mast cells and eosinophils. These important pro-asthmatic mediators bind to cysteinyl leukotriene (CysLT) receptors. The CysLT type-1 (CysLT1) receptor is found in the human airway (including airway smooth muscle cells and airway macrophages) and on other pro-inflammatory cells (including eosinophils and certain myeloid stem cells). CysLTs have been correlated with the pathophysiology of asthma and allergic rhinitis. In asthma, leukotriene-mediated effects include bronchoconstriction, mucous secretion, vascular permeability, and eosinophil recruitment. In allergic rhinitis, CysLTs are released from the nasal mucosa after allergen exposure during both early- and late-phase reactions and are associated with symptoms of allergic r hinitis. Intranasal challenge with CysLTs has been shown to increase nasal airway resistance and symptoms of nasal obstruction.
5.2 Pharmacodynamic properties
Bilastine
Highly selective for H1 receptors, but has little or no affinity for other receptors. In vitro binding affinity of Maxstine M to H1 receptors was 3-fold higher than that of cetirizine and 5-fold higher than that of fexofenadine Potent and lasting antihistamine action at least similar to cetirizine and superior to fexofenadine (4 to 11 times). Rapid onset of action and possesses antihistaminic as well as anti-allergic properties Maxstine M is a “Non- brain-penetrating antihistamine” which has brain H1 receptor occupancy (H1RO) is nearly 0%.
Montelukast
Montelukast is an orally active compound which binds with high affinity and selectivity to the CysLT1 receptor. In clinical studies, Montelukast inhibits bronchoconstriction due to inhaled LTD4 at doses as low as 5 mg. Bronchodilation was observed within 2 hours of oral administration. The bronchodilation effect caused by a b agonist was additive to that caused by Montelukast. Treatment with Montelukast inhibited both early- and late phase bronchoconstriction due to antigen challenge. Montelukast, compared with placebo, decreased peripheral blood eosinophils in adult and paediatric patients. In a separate study, treatment with Montelukast significantly decreased eosinophils in the airways (as measured in sputum) and in peripheral blood while improving clinical asthma control.
5.3 Pharmacokinetic properties
Bilastine
Absorption
Bilastine is rapidly absorbed after oral administration with a time to maximum plasma concentration of around 1.3 hours. No accumulation was observed. The mean value of Bilastine oral bioavailability is 61%.
Distribution
In vitro and in vivo studies have shown that Bilastine is a substrate of P-gp (“Interaction with ketoconazole, erythromycin and Diltiazem”) and OATP (“Interaction with grapefruit juice”). Bilastine does not appear to be a substrate of the transporter BCRP or renal transporters OCT2, OAT1 and OAT3. Based on in vitro studies, Maxstine M is not expected to inhibit the following transporters in the systemic circulation : P-gp, MRP2, BCRP, BSEP, OATP1B1, OATP1B3, OATP2B1, OAT1, OAT3, OCT1, OCT2, and NTCP, since only mild inhibition was detected for P-gp, OATP2B1 and OCT1, with an estimated IC50 ≥ 300 µM, much higher than the calculated clinical plasma Cmax and therefore these interactions will not be clinically relevant. However, based on these results inhibition by Bilastine of transporters present in the intestinal mucosa, e.g. P-gp, cannot be excluded. At therapeutic doses Bilastine is 84-90% bound to plasma proteins.
Biotransformation
Bilastine did not induce or inhibit activity of CYP450 isoenzymes in in vitro studies.
Elimination
In a mass balance study performed in healthy adult volunteers, after administration of a single dose of 20 mg 14C-Bilastine, almost 95% of the administered dose was recovered in urine (28.3%) and faeces (66.5%) as unchanged Bilastine, confirming that Bilastine is not significantly metabolized in humans. The mean elimination half-life calculated in healthy volunteers was 14.5 h.
Montelukast
Absorption
Montelukast is rapidly absorbed following oral administration. For the 10 mg film-coated tablet, the mean peak plasma concentration (C ) is achieved 3 hours (T ) after administration in max max adults in the fasted state. The mean oral bioavailability is 64%. The oral bioavailability and C are not influenced by a standard meal. Safety and efficacy were demonstrated in clinical max trials where the 10 mg film-coated tablet was administered without regard to the timing of food ingestion. For the 5 mg chewable tablet, the C is achieved in 2 hours after administration in adults in the fasted state. The mean oral bioavailability is 73% and is decreased to 63% by a standard max meal.
Distribution
Montelukast is more than 99% bound to plasma proteins. The steady-state volume of distribution of Montelukast averages 8-11 litres. Studies in rats with radiolabelled Montelukast indicate minimal distribution across the blood-brain barrier. In addition, concentrations of radiolabelled material at 24 hours post-dose were minimal in all other tissues.
Biotransformation
Montelukast is extensively metabolised. In studies with therapeutic doses, plasma concentrations of metabolites of Montelukast are undetectable at steady state in adults and children. Cytochrome P450 2C8 is the major enzyme in the metabolism of Montelukast. Additionally, CYP 3A4 and 2C9 may have a minor contribution, although Itraconazole, an inhibitor of CYP 3A4, was shown not to change pharmacokinetic variables of Montelukast in healthy subjects that received 10 mg Montelukast daily. Based on in vitro results in human liver microsomes, therapeutic plasma concentrations of Montelukast do not inhibit cytochromes P450 3A4, 2C9, 1A2, 2A6, 2C19, or 2D6. The contribution of metabolites to the therapeutic effect of Montelukast is minimal.
Elimination
The plasma clearance of Montelukast averages 45 ml/min in healthy adults. Following an oral dose of radiolabelled Montelukast, 86% of the radioactivity was recovered in 5-day faecal collections and < 0.2% was recovered in urine. Coupled with estimates of Montelukast oral bioavailability, this indicates that Montelukast and its metabolites are excreted almost exclusively via the bile.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Bilastine
Non-clinical data with Bilastine reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential. In reproduction toxicity studies effects of Bilastine on the foetus (pre-and post-implantation loss in rats and incomplete ossification of cranial bones, sternebrae and limbs in rabbits) were only observed at maternal toxic doses. The exposure levels at the NOAELs are sufficiently in excess (> 30 fold) to the human exposure at the recommended therapeutic dose. In a lactation study, Bilastine was identified in the milk of nursing rats administered a single oral dose (20 mg/kg). Concentrations of Bilastine in milk were about half of those in maternal plasma. The relevance of those results for humans is unknown. In a fertility study in rats, Bilastine administered orally up to 1000 mg/kg/day did not induce any effect on female and male reproductive organs. Mating, fertility and pregnancy indices were not affected.
As seen in a distribution study in rats with determination of drug concentrations by autoradiography, Bilastine does not accumulate in the CNS.
Montelukast
In animal toxicity studies, minor serum biochemical alterations in ALT, glucose, phosphorus and triglycerides were observed which were transient in nature. The signs of toxicity in animals were increased excretion of saliva, gastro-intestinal symptoms, loose stools and ion imbalance. These occurred at dosages which provided > 17-fold the systemic exposure seen at the clinical dosage. In monkeys, the adverse effects appeared at doses from 150 mg/kg/day (> 232-fold the systemic exposure seen at the clinical dose). In animal studies, Montelukast did not affect fertility or reproductive performance at systemic exposure exceeding the clinical systemic exposure by greater than 24-fold. A slight decrease in pup body weight was noted in the female fertility study in rats at 200 mg/kg/day (> 69 fold the clinical systemic exposure). In studies in rabbits, a higher incidence of incomplete ossification, compared with concur rent control animals, was seen at systemic exposure > 24-fold the clinical systemic exposure seen at the clinical dose. No abnormalities were seen in rats. Montelukast has been shown to cross the placental barrier and is excreted in breast milk of animals. 2 2 No deaths occurred following a single oral administration of Montelukast sodium at doses up to 5000 mg/kg in mice and rats (15,000 mg/m and 30,000 mg/m in mice and rats, respectively), the maximum dose tested. This dose is equivalent to 25,000 times the recommended daily adult human dose (based on an adult patient weight of 50 kg). Montelukast was determined not to be phototoxic in mice for UVA, UVB or visible light spectra at doses up to 500 mg/kg/day (approximately > 200-fold based on systemic exposure). Montelukast was neither mutagenic in in vitro and in vivo tests nor tumorigenic in rodent species.
7.0 Description
Bilastine is a novel, benzimidazole–piperidine derivative that is a highly selective second-generation H1 antihistamine. Montelukast sodium, the active ingredient in Montelukast sodium tablets, is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl leukotriene CysLT1 receptor.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
Alu-Alu blister strip of 10 tablets
8.4 Storage & handing instructions
Store below 30°C, protected from light & moisture.
Keep out of reach of children.
Do not use this medicine after the expiry date which is stated on the carton and the blisters after EXP. The expiry date refers to the last day of that month
9.0 Patient counselling information
• Do not take this medicine, if you are allergic to Bilastine or Montelukast.
• Signs of an allergic reaction include a rash, itching or shortness of breath.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
• Be alert for changes in behaviour or new neuropsychiatric symptoms when taking Montelukast Sodium. If changes in behaviour are observed, or if new NP symptoms or suicidal thoughts and/or behaviour occur, advise patients to discontinue Montelukast Sodium and contact a healthcare provider immediately.
• If you have any further questions, ask your doctor or pharmacist.
10.0 Details of manufacturer
Synokem Pharmaceuticals Ltd.
Plot No. 56-57, Sector-6A, I.I.E. (SIDCUL), Ranipur (BHEL), Haridwar - 249 403 (Uttarakhand)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for your child. Do not pass it on to others. It may harm them, even
- if their signs of illness are the same as your child’s.
- If your child gets any side effects, talk to your doctor or pharmacist. This includes any possible side
- effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What MAXSTINE M is and what it is used for
2. What you need to know before you take MAXSTINE M
3. How to take MAXSTINE M
4. Possible side effects
5. How to store MAXSTINE M
6. Contents of the pack and other information
1. What MAXSTINE M is and what it is used for
MAXSTINE M contains the active substance bilastine which is an antihistamine and Montelukast, a leukotriene receptor antagonist that blocks the substance called leukotrienes. Leukotrienes can cause the narrowing and swelling of airways in your lungs and also cause allergy symptoms. By blocking leukotrienes, this medicine improves asthma symptoms, helps control asthma and improves seasonal allergy symptoms (also known as hay fever or seasonal allergic rhinitis).
MAXSTINE M is used to relieve the symptoms of hayfever (sneezing, itchy, runny, blocked-up nose and red and watery eyes) and other forms of allergic rhinitis.
MAXSTINE M -20 mg tablet is indicated in adults and adolescents (15 years and over)
2. What you need to know before you take MAXSTINE M 20 mg tablets
Do not take MAXSTINE M
if you are allergic to bilastine, montelukast or any of the other ingredients of this medicine
Warnings and precautions
- Talk to your doctor or pharmacist before using MAXSTINE M if you have moderate or severe renal impairment and in addition you are taking other medicines (see “Other medicines and MAXSTINE M”).
- If your asthma or breathing gets worse, tell your doctor immediately.
- Oral MAXSTINE M Tablets is not meant to treat acute asthma attacks. If an attack occurs, follow the instructions your doctor has given you. Always have with you, your inhaled rescue medicine for asthma attack.
- Any patient on anti-asthma medicines should be aware that if you develop a combination of symptoms such as a flu-like illness, pins and needles or numbness of arms or legs, worsening of pulmonary symptoms, and/or rash, you should consult your doctor.
- You should not take acetyl-salicyclic acid (aspirin) or anti-inflammatory medicines (also known as non-steroidal anti-inflammatory drugs or NSAIDS) if they make your asthma worse.
- It is important that you or your child takes all asthma medicines as prescribed by your doctor.
MAXSTINE M should not be used in place of other asthma medications your doctor has prescribed for you.
Patients should be aware that various neuropsychiatric events (for example behaviour and mood related changes) have been reported in adults, adolescents and children with Montelukast (see section 4). If you develop(s) such symptoms while taking MAXSTINE M tablets, you should consult your doctor.
Children
Do not give this medicine to children under 15 years of age.
Do not exceed the recommended dose. If symptoms persist, consult your doctor.
Other medicines and MAXSTINE M
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
In particular, please discuss with your doctor if you are taking any of the following medicines:
Ketoconazole (an antifungal medicine)
Erythromycin (an antibiotic)
Diltiazem (to treat angina)
Cyclosporine (to reduce the activity of your immune system, thus avoiding transplant rejection or reducing disease activity in autoimmune and allergic disorders, such as psoriasis, atopic dermatitis or rheumatoid arthritis)
Ritonavir (to treat AIDS)
Rifampicin (an antibiotic)
Phenobarbital (used for treatment of epilepsy)
Phenytoin (used for treatment of epilepsy)
Rifampicin (used to treat tuberculosis and some other infections)
Gemfibrozil (used for treatment of high lipid levels in plasma).
MAXSTINE M with food, drink
These tablets should not be taken with food or with grapefruit juice or other fruit juices, as this will decrease the effect of bilastine. To avoid this, you can:
take the tablet and wait for one hour before taking food or fruit juice or
if you have taken food or fruit juice, wait for two hours before taking the tablet.
Pregnancy, breast-feeding and fertility
There are no or limited amount of data from the use of bilastine in pregnant women and during breast-feeding and on the effects on fertility.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
It has been demonstrated that bilastine 20 mg does not affect the driving performance in adults. However, the response from each patient to the medicine may be different. Certain side effects (such as dizziness and drowsiness) that have been reported with montelukast may affect some patient’s ability to drive or operate machinery, therefore caution is advised.
3. How to take MAXSTINE M
Always take this medicine exactly as your doctor or pharmacist have told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose in adults and adolescents aged 15 years and over, is 1 tablet (20/10 mg) a day.
- The tablet is for oral use.
- The tablet must be taken one hour before or two hours after intake of food or fruit juice (see section 2, MAXSTINE M with food, drink”).
- Swallow your tablet with a glass of water.
Regarding the duration of treatment, your physician will determine the type of disease you are suffering from and will determine for how long you should take MAXSTINE M.
If you take more MAXSTINE M than you should
If you, or anyone else, have taken too many MAXSTINE M tablets, contact your doctor or pharmacist immediately or go to the emergency department of your nearest hospital.
Please remember to take this medicine pack or this leaflet with you.
If you forget to take MAXSTINE M
Do not take a double dose to make up for a forgotten dose.
If you forget to take your dose on time, take it as soon as possible, and then go back to your regular dosing schedule.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you experience symptoms of an allergic reaction the signs of which may include difficulty in breathing, dizziness, collapsing or losing consciousness, swelling of your face, lips, tongue or throat, and/or
swelling and redness of the skin, stop taking the medicine and seek urgent medical advice straight away.
Bilastine
Other side effects that may be experienced in adults and adolescents are:
Common: may affect up to 1 in 10 people
- Headache
- Drowsiness
- Uncommon: may affect up to 1 in 100 people
- abnormal ECG heart tracing
- blood tests which show changes in the way the liver is working
- dizziness
- stomach pain
- tiredness
- increased appetite
- irregular heartbeat
- increased weight
- nausea (the feeling of being sick)
- anxiety
- dry or uncomfortable nose
- belly pain
- diarrhoea
- gastritis (inflammation of the stomach wall)
- vertigo (a feeling of dizziness or spinning)
- feeling of weakness
- thirst
- dyspnoea (difficulty in breathing )
- dry mouth
- indigestion
- itching
- cold sores (oral herpes)
- fever
- tinnitus (ringing in the ears)
- difficulty in sleeping
- blood tests which show changes in the way kidney is working
- blood fats increased
Frequency not known: cannot be estimated from the available data
- palpitations (feeling your heart beat)
- tachycardia (fast heart beat)
- vomiting
Side effects that may be experienced in children are:
Common: may affect up to 1 in 10 people
- rhinitis (nasal irritation)
- allergic conjunctivitis (eye irritation)
- headache
- stomach pain (abdominal /upper abdominal pain)
Uncommon: may affect up to 1 in 100 people
- eye irritation
- dizziness
- loss of consciousness
- diarrhoea
- nausea (the feeling of being sick)
- lip swelling
- eczema
- urticaria (hives)
- fatigue
Montelukast
Tell your doctor right away if you get one or more of these symptoms:
In asthmatic patients treated with montelukast, very rare cases of a combination of symptoms such as flu like illness, pins and needles or numbness of arms and legs, worsening of pulmonary symptoms and/or rash (Churg - Strauss syndrome) have been reported.
Stop taking this medicine and seek medical attention immediately if you get one or more of these symptoms:
- serious allergic reactions (Hypersensitivity) including potentially life-threatening reactions including, swelling of the face, lips, tongue and/or throat which may cause difficulty in breathing or swallowing.
- swelling (inflammation with eosinophilia) of the lungs
- suicidal thoughts and actions
- hepatitis (swelling of your liver) which can cause abdominal pain, nausea, vomiting, producing dark urine, jaundice (yellowing of the skin and whites of the eyes.
- severe skin reactions (erythema multiforme) and tender red lumps under the skin commonly on your shins (erythema nodosum) that may occur without warning.
In clinical studies with Montelukast 10 mg tablets, the most commonly reported side effects (occurring in more than 1 in 100, or less than 1 in 10 treated patients) thought to be related to this medicine were:
- Abdominal pain
- Headache
In clinical studies with montelukast 5 mg chewable tablets, the most commonly reported side effects (occurring in at least 1 of 100 patients and less than 1 of 10 paediatric patients treated) thought to be related to this medicine was:
Headache
These were usually mild and occurred at a greater frequency in patients treated with montelukast than placebo (a pill containing no medication).
Additionally, while the medicine has been on the market, the following have been reported:
Very common side effects (may affect more than 1 in 10 people):
- Infection of the throat or the airways going into the lungs.
Common side effects (may affect up to 1 in 10 people):
- Diarrhoea
- Nausea
- Vomiting
- Rash
- abnormal liver function
- fever
Uncommon side effects (may affect up to 1 in 100 people):
- nose bleed
- dry mouth
- indigestion
- bruising
- itching
- hives
- joint and / or muscle pain, muscle cramps
- pins and needles/ numbness
- weakness/ tiredness
- feeling unwell
- swelling
- feeling dizzy
- feeling drowsy
- seizure (having a fit)
- Behaviour and mood related changes, such as:
- dream abnormalities
- nightmares
- trouble sleeping
- sleep walking
- irritability
- feeling anxious
- restlessness
- agitation including aggressive behaviour or hostility
- depression
Rare side effects (may affect up to than 1 in 1,000 people):
- increased bleeding tendency
- tremor
- palpitations
- disturbance in attention
- memory impairment
- Serious allergic reaction which causes swelling of the face, lips, mouth, tongue or throat (angioedema)
Very rare side effects (may affect up to 1 in 10,000 people):
hallucinations (seeing things which are not there)
disorientation
stuttering
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store MAXSTINE M
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and the blisters after EXP.
Store below 30°C. Protected from light & moisture.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What MAXSTINE M contains
Each film coated bilayered tablet contains:
Bilastine 20 mg
Montelukast 10 mg
Excipients q.s.
© Zuventus Healthcare Ltd., 2020. All rights reserved.