Maxtra® Cold + Tablets
Therapy Area
Respiratory
1.0 Generic Name
Paracetamol, Phenylephrine Hydrochloride, Diphenhydramine Hydrochloride & Caffeine Tablets
2.0 Qualitative and quantitative composition
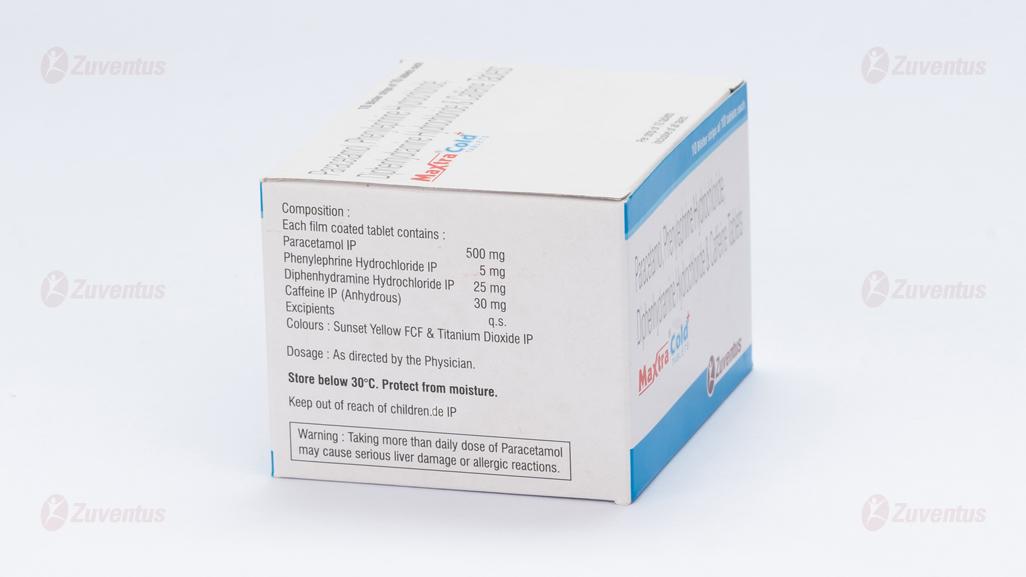
Each film coated tablet contains:
Paracetamol IP 500 mg
Phenylephrine Hydrochloride IP 5 mg
Diphenhydramine Hydrochloride IP 25 mg
Caffeine IP (Anhydrous) 30 mg
Excipients q.s.
Colours: Sunset Yellow FCF & Titanium Dioxide IP
3.0 Dosage form and strength
Dosage Form: Tablet.
Dosage Strength: Paracetamol 500 mg, Phenylephrine 5 mg, Diphenhydramine 25 mg and Caffeine 30 mg per tablet.
4.0 Clinical particulars
4.1 Therapeutic indication
For treatment of fever associated with cold, nasal congestion in adults.
4.2 Posology and method of administration
For oral administration.
Adults: 1 tablet to be administered 3 to 4 times daily.
Doses of Individual Ingredients:
As a nasal decongestant, Phenylephrine Hydrochloride may be given in usual oral doses of 10 mg every four hours (up to a maximum of 60 mg daily) or 12 mg up to four times daily.
The usual oral dose of Paracetamol is 0.5 to 1 g every 4 to 6 hours up to a maximum of 4 g daily.
Diphenhydramine Hydrochloride is given in usual oral doses of 25 to 50 mg three or four times daily.
The recommended dosage of Caffeine as an adjuvant analgesic is from 15 to 65 mg/dose.
Do not exceed the recommended dose, as paracetamol may cause liver toxicity with overdose. Maxtra Cold Plus Tablets should be preferably administered with or after food. The tablet should be swallowed whole with water or, as prescribed by the physician.
4.3 Contraindications
Maxtra Cold Plus Tablets are contraindicated in the following:
- Known hypersensitivity to paracetamol or to phenylephrine or to caffeine or to diphenhydramine or to any component of the formulation.
- In patients who have been treated with monoamine oxidase (MAO) inhibitors within the last 14 days.
- In patients who are currently receiving other sympathomimetic drugs.
- Cardiovascular disorders.
- In patients with peripheral vascular insufficiency.
- Severe hepatic and renal impairment.
- In patients with hyperthyroidism.
- In patients with glaucoma.
- In patients with prostate problems (such as hypertrophy).
- Pheochromocytoma.
4.4 Special warnings and precautions for use
Paracetamol
Significant overdose of paracetamol can lead to hepatotoxicity in some patients. Thus, do not exceed the recommended dose. The hazards of overdose are greater in those with non-cirrhotic alcoholic liver disease.
Do not take with any other paracetamol-containing products, so as to avoid the chances of overdose.
Care is advised in the administration of paracetamol to patients with severe renal or severe hepatic impairment.
Chronic heavy alcohol abusers may be at increased risk of liver toxicity from excessive paracetamol use.
Phenylephrine
Sympathomimetic amines should be used with caution in patients with hypertension, diabetes mellitus, heart disease (angina), peripheral vascular disease, increased intraocular pressure, hyperthyroidism, or prostatic hypertrophy.
Phenylephrine should not be used with other sympathomimetics (such as decongestants, appetite suppressants, and amphetamine-like psychostimulants).
Sympathomimetics may act as cerebral stimulants giving rise to insomnia, nervousness, hyperpyrexia, tremor, and epileptiform convulsions.
Diphenhydramine
Subjects with moderate to severe renal or hepatic dysfunction should exercise caution while using diphenhydramine.
Diphenhydramine should not be taken by individuals with narrow-angle glaucoma or symptomatic prostatic hypertrophy.
Caution should be exercised in patients with glaucoma and urinary retention.
Should be used with caution in patients with myasthenia gravis or seizure disorders, bronchitis or chronic obstructive pulmonary disease (COPD).
Tolerance may develop with continuous use.
Diphenhydramine may exacerbate tinnitus in existing tinnitus sufferers.
Caffeine
Excessive intake of caffeine (e.g., coffee, tea and some canned drinks) should be avoided while taking this product.
Caffeine should be administered with caution in patients with a history of peptic ulcers.
Prolonged, high intake of caffeine may produce tolerance and habituation. Physical signs of withdrawal, such as headaches, irritation, nervousness, anxiety, and dizziness may occur upon abrupt discontinuation.
4.5 Drug interactions
Paracetamol
Cholestyramine: The rate of absorption of paracetamol is reduced by cholestyramine. Therefore, cholestyramine should not be taken within one hour, if maximal analgesia is required.
Metoclopramide and Domperidone: The absorption of paracetamol is increased by metoclopramide and domperidone. However, concurrent use need not be avoided.
Warfarin: The anti-coagulant effect of warfarin and other coumarins may be enhanced by prolonged regular use of paracetamol with increased risk of bleeding; occasional doses have no significant effect.
Chloramphenicol: Concurrent administration of paracetamol and chloramphenicol may markedly retard the elimination of chloramphenicol and thus, increases plasma concentration of chloramphenicol which leads to risk of its harmful effects. Monitoring of chloramphenicol plasma levels is recommended while combining paracetamol with chloramphenicol injection.
Alcohol, Anticonvulsants, and Isoniazid: Concomitant administration of alcohol, anticonvulsants, and isoniazid with paracetamol may increase risk of hepatotoxicity.
Phenylephrine MAO Inhibitors: Hypertensive interactions occur between sympathomimetic amines such as phenylephrine and MAO inhibitors, thus concomitant use is contraindicated.
Sympathomimetic Amines: Concomitant use of phenylephrine with other sympathomimetic amines can increase the risk of cardiovascular side effects.
Beta-Blockers and Other Antihypertensives (Including Debrisoquine, Guanethidine, Reserpine, and Methyldopa): Phenylephrine may reduce the efficacy of beta-blocking drugs and antihypertensive drugs. The risk of hypertension and other cardiovascular side effects may be increased.
Tricyclic Antidepressants (Amitriptyline): Concomitant use of phenylephrine with amitriptyline may increase the risk of cardiovascular side effects.
Ergot Alkaloids (Ergotamine and Methylsergide): Concomitant use of phenylephrine with these drugs increases risk of ergotism.
Digoxin and Cardiac Glycosides: Co-administration of phenylephrine with these drugs increases risk of irregular heartbeat or heart attack.
Diphenhydramine Drugs Acting on Central Nervous System (CNS): Diphenhydramine may potentiate the effects of alcohol, codeine, antihistamines and other CNS depressant drugs.
Anticholinergic Agents: As diphenhydramine possesses some antimuscarinic activity, the effects of anticholinergics (e.g., tricyclic antidepressants and atropine) may be potentiated by diphenhydramine. This may result in tachycardia, dry mouth, gastrointestinal disturbances (e.g., colic), urinary retention and headache.
MAO Inhibitors: MAO inhibitors prolong and intensify the anticholinergic effects of diphenhydramine. Thus, diphenhydramine should be used with caution with MAO inhibitors or within 2 weeks of stopping MAO inhibitor.
Drugs Metabolized by CYP 2D6: Diphenhydramine is an inhibitor of the cytochrome P450 isoenzyme CYP2D6. Therefore, there is a potential for interaction with drugs which are primarily metabolised by CYP2D6, such as metoprolol and venlafaxine.
Caffeine
Drugs Metabolized by Cytochrome P450 1A2: Cytochrome P450 1A2 (CYP1A2) is known to be the major enzyme involved in the metabolism of caffeine. Therefore, caffeine has the potential to interact with drugs that are substrates for CYP1A2, inhibit CYP1A2, or induce CYP1A2. Lower doses of caffeine may be needed following co-administration of drugs which are reported to decrease caffeine elimination (e.g., cimetidine and ketoconazole) and higher caffeine doses may be needed following co-administration of drugs that increase caffeine elimination (e.g., phenobarbital and phenytoin). Caffeine may also increase the metabolism of other drugs such as phenobarbital and aspirin.
Ephedrine: Caffeine and ephedrine are both CNS stimulant drugs. Co-administration of caffeine with ephedrine might cause too much stimulation and sometimes serious side effects and heart problems. Do not administer caffeine-containing products and ephedrine at the same time.
Theophylline: Caffeine works similar to theophylline. Inter-conversion between caffeine and theophylline has been reported. Caffeine may decrease metabolism and excretion of theophylline and thus, might increase the effects and side effects of theophylline. The concurrent use of these drugs is not recommended.
Beta-Adrenergic Agonists: Caffeine may enhance the cardiac inotropic effects of beta-adrenergic stimulating agents. Co-administration of caffeine with these drugs should be avoided, as it might cause serious problems including increased heart rate and high blood pressure.
Disulfiram: Co-administration of caffeine and disulfiram may lead to a substantial decrease in caffeine clearance. Co-administration of caffeine with disulfiram might increase the effects and side effects of caffeine including jitteriness, hyperactivity, and irritability.
Quinolone Antibiotics: Caffeine accumulation may occur when products or foods containing caffeine are consumed concomitantly with quinolones such as ciprofloxacin. Co-administration of these antibiotics with caffeine can increase the risk of side effects including jitteriness, headache, increased heart rate, and other side effects.
4.6 Use in special populations
Pregnant Women
The safety of this formulation during pregnancy has not been established. Epidemiological studies in human pregnancy have shown no ill effects due to paracetamol used in the recommended dosage. There is a possible association of fetal abnormalities with first trimester exposure to phenylephrine. In addition, there is a potential for increased uterine contractility and vasoconstriction, with the possibility of fetal hypoxia. Phenylephrine may also reduce placental perfusion, thus, it should not be used in patients with a history of pre-eclampsia. The half-life of caffeine is prolonged when used during pregnancy. Caffeine is not recommended for use during pregnancy due to the possible increased risk of lower birth weight and spontaneous abortion associated with its consumption. Diphenhydramine crosses the placental barrier and has been reported to cause jaundice and extrapyramidal symptoms in infants whose mothers received the drug during pregnancy. Use of sedating antihistamines during the third trimester of pregnancy may result in reactions in the newborn or premature neonates. Thus, diphenhydramine is not recommended during pregnancy. Use of Maxtra Cold Plus Tablets should be avoided during pregnancy.
Lactating Women
Paracetamol and phenylephrine are excreted in breast milk, but not in a clinically significant amount. Caffeine appears in breast milk. Caffeine in breast milk may potentially have a stimulating effect on breast fed infants. Irritability and poor sleeping pattern in the infant have been reported. Diphenhydramine has been detected in breast milk. If administered during breast feeding there is an increased risk of adverse effects of antihistamine, such as unusual excitation or irritability in infants. Thus, diphenhydramine hydrochloride is not recommended for use during lactation in nursing mothers. Due to the caffeine and diphenhydramine content of this formulation, Maxtra Cold Plus Tablets should not be administered to a nursing mother. Accordingly, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Paediatric Patients
Safety and effectiveness of this formulation in paediatric patients below 12 years of age have not been established. Thus, Maxtra Cold Plus Tablets are not recommended in this age group.
Geriatric Patients
Elderly patients with normal renal and hepatic function should be given the same dose as recommended for adults. Side effects are more likely to occur in the elderly. The risk of toxic reactions with this formulation may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
4.7 Effects on ability to drive and use machines
Diphenhydramine content of this formulation has a major influence on the ability to drive and use machines. It may cause drowsiness or sedation soon after the dose has been taken or up to 8 hours of ingestion. Diphenhydramine may also cause dizziness, blurred vision, cognitive and psychomotor impairment which may adversely affect patient's ability to concentrate and react. Thus, while on this medicine, patients are advised not to drive a vehicle or operate machinery.
4.8 Undesirable effects
Paracetamol
Adverse effects of paracetamol are rare. However, hypersensitivity including skin rash and fixed drug eruption (FDE) may occur. There have been reports of blood dyscrasias including thrombocytopenic purpura, methaemoglobenemia and agranulocytosis, but these were not necessarily related to paracetamol. Overdosage with paracetamol can result in severe hepatotoxicity and sometimes acute renal tubular necrosis. If there is a pre-existing liver insufficiency, paracetamol can be hepatotoxic even in normal dosage. Increased levels of aspartate aminotransferase and hepatic transaminases may occur. Nausea, vomiting, abdominal pain, diarrhea, constipation, dyspepsia, dry mouth, heartburn have also been reported commonly with the use of paracetamol.
Phenylephrine
Phenylephrine may elevate blood pressure with headache, vomiting and rarely palpitations, tachycardia or reflex bradycardia, tingling and coolness of the skin. There have been rare reports of allergic reactions.
Diphenhydramine
Diphenhydramine may cause drowsiness, dizziness, gastrointestinal disturbance, dry mouth, difficulty in urination or blurred vision. Less frequently diphenhydramine may also cause palpitations, tremor, convulsions or parasthesia. Hypersensitivity reactions have been reported with the use of diphenhydramine, in particular, skin rashes, erythema, urticaria and angioedema.
Caffeine
Caffeine may cause CNS adverse effects such as insomnia, dizziness, restlessness, excitability, anxiety, irritability, nervousness and mild delirium. Caffeine may also cause gastrointestinal adverse effects such as nausea, vomiting and gastric irritation. High doses of caffeine can cause tremor and palpitations.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.8 Overdose
Paracetamol
Symptoms: Ingestion of 5 gram or more of paracetamol may lead to liver damage. Symptoms of paracetamol overdose in the first 24 hours are pallor, nausea, vomiting, anorexia and abdominal pain. Liver damage may become apparent 12 to 48 hours after ingestion. Abnormalities of glucose metabolism and metabolic acidosis may occur. In severe poisoning, hepatic failure may progress to encephalopathy, hemorrhage, hypoglycaemia, cerebral edema, and death. Acute renal failure with acute tubular necrosis, strongly suggested by loin pain, hematuria and proteinuria, may develop even in the absence of severe liver damage. Cardiac arrhythmias and pancreatitis have been reported.
Treatment: Immediate treatment is essential in the management of paracetamol overdose. Treatment with activated charcoal should be considered if the overdose has been taken within 1 hour. Plasma paracetamol concentration should be measured at 4 hours or later after ingestion (earlier concentrations are unreliable). Treatment with N-acetylcysteine (FDA approved antidote) may be used up to 24 hours after ingestion of paracetamol. However, the maximum protective effect is obtained up to 8 hours post ingestion.
Phenylephrine
Symptoms: Overdose symptoms may include hypertension and possibly reflex bradycardia. In severe cases confusion, hallucinations, seizures, and arrhythmias may occur.
Treatment: Treatment measures include early gastric lavage and symptomatic and supportive measures. The hypertensive effects may be treated with an α-receptor blocking agent (such as phentolamine mesylate, 6 to 10 mg) given intravenously, and the bradycardia treated with atropine, preferably only after the pressure has been controlled.
Diphenhydramine
Symptoms: The symptoms of diphenhydramine overdose may include drowsiness, hyperpyrexia and anticholinergic effects. With higher doses, and particularly in children, symptoms of CNS excitation including hallucinations and convulsions may appear; with massive doses, coma or cardiovascular collapse may follow.
Treatment: Treatment of overdose should be symptomatic and supportive. Measures to promote rapid gastric emptying (ipecac-induced emesis or gastric lavage) and, in cases of acute poisoning, the use of activated charcoal may be useful. Seizures may be controlled with diazepam or thiopental sodium. The intravenous use of physostigmine may be efficacious in antagonising severe antichlolinergic symptoms.
Caffeine
Symptoms: Common features include CNS stimulation; anxiety, nervousness, restlessness, insomnia, excitement, muscle twitching, confusion, convulsions. Cardiac symptoms include tachycardia, cardiac arrhythmia. Gastric symptoms include abdominal or stomach pains. Other symptoms of caffeine overdose include diuresis and facial flushing.
Treatment: Treatment of caffeine overdose is primarily symptomatic and supportive. Diuresis should be treated by maintaining fluid and electrolyte balance and CNS symptoms can be controlled by intravenous administration of diazepam.
5.0 Pharmacological properties
5.1 Mechanism of Action
Paracetamol - Analgesic and Antipyretic
Analgesic Effect: The mechanism of analgesic action of paracetamol has not been fully determined. Paracetamol may act predominantly by inhibiting prostaglandin synthesis in the central nervous system (CNS) and to a lesser extent, through a peripheral action by blocking pain-impulse generation. The peripheral action may also be due to inhibition of prostaglandin synthesis or inhibition of the synthesis or actions of other substances that sensitise pain receptors to mechanical or chemical stimulation.
Antipyretic Effect: Paracetamol produces antipyretic effect by acting centrally on the hypothalamic heat-regulation center to produce peripheral vasodilation resulting in increased blood flow through the skin, sweating and heat loss. The central action involves inhibition of prostaglandin synthesis in the hypothalamus.
Phenylephrine - Sympathomimetic Nasal Decongestant
Phenylephrine is a nasal decongestant with a potent postsynaptic α-receptor agonist activity. Dilated blood vessels can cause nasal blocks or stuffy nose. Phenylephrine shrinks blood vessels in the nasal passages and thus, reduces nasal congestion. A direct action at the receptors accounts for the greater part of its effects, whereas only a small part of effect is due to its ability to release norepinephrine. Sympathomimetic amines, such as phenylephrine, act on α-adrenergic receptors of the respiratory tract to produce vasoconstriction effect. This result in temporarily reduction of swelling associated with inflammation of the mucous membranes lining the nasal and sinus passages. This allows the free drainage of the sinusoidal fluid from the sinuses. In addition to reducing mucosal lining swelling, phenylephrine also suppresses the production of mucous, therefore preventing a buildup of fluid within the nasal cavities.
Diphenhydramine: Antihistamine
Antihistaminic Effect: Diphenhydramine is a potent H1-receptor antagonist. It diminishes or abolishes the actions of histamine by competitive reversible blockade of histamine H1-receptor sites on effector cells. Anti-histamine action occurs by blocking the spasmogenic and congestive effects of histamine thus, preventing, but not reversing responses mediated by histamine.
Anticholinergic Activity: Diphenhydramine blocks the parasympathetic activity which stimulates mucus gland secretion, thereby causing dryness of nasal mucosa and reducing nasal discharge.
Caffeine: CNS Stimulant
Caffeine stimulates all levels of the CNS, although its cortical effects are milder and of shorter duration than those of amphetamines. Caffeine constricts cerebral vasculature with an accompanying decrease in the cerebral blood flow and in the oxygen tension of the brain. It is believed that caffeine helps to relieve headache by providing more rapid onset of action and/or enhanced pain relief with lower doses of analgesic.
5.2 Pharmacodynamic properties
Paracetamol
Paracetamol is a centrally acting analgesic and antipyretic agent.
Phenylephrine
Phenylephrine is an orally active sympathomimetic amine and exerts a decongestant action on the nasal mucosa.
Diphenhydramine
Diphenhydramine is a potent first generation antihistaminic agent. Diphenhydramine produces antiallergic effect by blocking H1 receptor on the effector cell surface. Diphenhydramine also possesses anticholinergic properties. Diphenhydramine relieves runny nose, sneezing, itchy and watery eyes, and itchy throat.
Caffeine
Caffeine is a CNS stimulating agent. Caffeine produces wakefulness and increased mental activity/ alertness.
5.3 Pharmacokinetic properties
Paracetamol
Paracetamol is readily absorbed from the GIT with peak plasma concentrations occurring about 30 minutes to 2 hours after ingestion. It is metabolized in the liver and excreted in the urine mainly as the glucuronide and sulphate conjugates. Less than 5% is excreted as unchanged paracetamol. The elimination half-life varies from about 1 to 4 hours. Plasma protein binding is negligible at usual therapeutic concentrations, but increases with increasing concentrations.
Phenylephrine
Phenylephrine is rapidly absorbed from the GIT and undergoes first-pass metabolism by MAO in the gut and liver. As a consequence, systemic bioavailability of oral route is only about 40%. Following oral administration, peak plasma concentration is achieved in 1 to 2 hours. Distribution in the brain appears to be minimal. Following absorption, the drug is extensively metabolised in the liver as the sulphate conjugate. Both phenylephrine and its metabolites are excreted in the urine. The mean plasma half-life is in the range 2 to 3 hours.
Diphenhydramine
Diphenhydramine is well absorbed from the gut following oral administration. Following a 50 mg oral dose, peak plasma levels of diphenhydramine are reached between 2 to 2.5 hours. Diphenhydramine is widely distributed throughout the body, including the CNS. Following a 50 mg oral dose of diphenhydramine, the volume of distribution is in the range 3.3 to 6.8 l/kg. Plasma protein binding is about 78%. Diphenhydramine undergoes extensive first pass metabolism. Plasma clearance of a 50 mg oral dose of diphenhydramine ranges from 600 to 1300 ml/min and the terminal elimination half-life ranges from 3.4 to 9.3 hours. Little unchanged drug is excreted in the urine.
Caffeine
After oral administration, caffeine is completely and rapidly absorbed with peak plasma concentrations occurring between 5 and 90 minutes (in fasting state). There is no evidence of presystemic metabolism. Caffeine distributes into all body fluids. The mean plasma protein binding of caffeine is 35%. Caffeine is metabolised almost completely via oxidation, demethylation, and acetylation, and is excreted in the urine as 1-methyluric acid, 1- methylxanthine, 7-methylxanthine, 1,7-dimethylxanthine (paraxanthine). Minor metabolites include 1-methylacrylic acid and 5-acethylamine-6-formylamine-3-methyluracil (AFMU). In adults, elimination is almost entirely by hepatic metabolism. Marked individual variability occurs in the rate of elimination. The mean plasma elimination half-life is 4.9 hours, with a range of 1.9 to 12.2 hours.
6.0 Nonclinical properties
6.1 Animal Toxicology
Paracetamol
Preclinical data reveal no special hazard for humans with paracetamol based on conventional studies of single and repeated dose toxicity, genotoxicity and carcinogenicity. Studies for the evaluation of toxicity to reproduction and development are not available.
Phenylephrine
Toxicity: LD50 values for phenylephrine have been determined in several species by various routes of administration. In Wistar rats, the LD50 value by intraperitoneal injection was 17 mg/kg and by subcutaneous injection was 33 mg/kg. The LD50 values in male Swiss mice were 89 mg/kg (intraperitoneal) and 22 mg/kg (subcutaneous). New Zealand rabbits had LD50 values of 0.5 mg/kg (intravenous), 7.2 mg/kg (intramuscular), and 22 mg/kg (subcutaneous).
Mutagenicity: Phenylephrine hydrochloride was not mutagenic in four tester strains of Salmonella typhimurium (TA100, TA1535, TA1537, and TA98) in the presence or absence of Aroclor 1254-induced male Sprague-Dawley rat or male Syrian hamster liver S9.
Carcinogenicity: In the mouse study, the mean daily dose (males and females) in the low dose animals was 133 mg/kg and in the high dose animals 270 mg/kg. The study demonstrated no evidence of carcinogenicity in rats and mice under the testing conditions employed.
Diphenhydramine
Mutagenesis: Diphenhydramine hydrochloride was not mutagenic in Salmonella typhimurium strains TA98, TA100, TA1535, or TA1537 when tested in either the presence or absence of exogenous metabolic activation. Exposure to this chemical did not induce trifluorothymidine (Tft) resistance in mouse L5178Y lymphoma cells with or without metabolic activation. In cytogenetic tests with cultured CHO cells, diphenhydramine hydrochloride induced chromosomal aberrations in the absence, but not the presence, of exogenous metabolic activation (S9); no induction of sister chromatid exchanges (SCEs) was observed in these cells with or without S9.
Carcinogenicity: Carcinogenesis studies were conducted by feeding diets containing USP-grade diphenhydramine hydrochloride to groups of F344/N rats and B6C3F1 mice of each sex. In these 2-year feed studies, there was equivocal evidence of carcinogenic activity of diphenhydramine hydrochloride for male F344/N rats, based on marginally increased incidences of uncommon brain neoplasms (astrocytomas or gliomas) and of alveolar/bronchiolar neoplasms. There was equivocal evidence of carcinogenic activity for female F344/N rats, based on a marginal increase in the incidence of pituitary gland adenomas. There was no evidence of carcinogenic activity for male or female B6C3F1 mice fed diets containing 156 or 313 ppm diphenhydramine hydrochloride. 12
Developmental Toxicity: Pregnant rats and rabbits were given diphenhydramine hydrochloride orally during the period of organogenesis at drug levels yielding about 3 to 4 and 15 to 19 mg/kg. Rats tolerated the drug well, and there was only a slight inhibitory effect on food intake and weight gain. Neither clinical reactions nor deaths occurred among the dams, and pregnancy was uneventful. Fetal parameters at term in the pups of treated dams were comparable to pups of control dams, and no malformations were induced. In rabbits, weight gain depression among the high dose dams was the only response elicited. As in the rats, fetal parameters were comparable between treated and control groups.
Caffeine
Carcinogenicity: In a 2-year study in Sprague-Dawley rats, caffeine (as caffeine base) administered in drinking water was not carcinogenic in male rats at doses up to 102 mg/kg or in female rats at doses up to 170 mg/kg (approximately 2.8 and 4.6 times, respectively, the daily dose of 360 mg caffeine on a mg/m2 basis). In an 18-month study in C57BL/6 mice, no evidence of tumorigenicity was seen at dietary doses up to 55 mg/kg (0.7 times the daily dose of 360 mg caffeine on a mg/m2 basis).
Mutagenesis: Caffeine (as caffeine base) increased the sister chromatid exchange (SCE) SCE/cell metaphase (exposure time dependent) in an in vivo mouse metaphase analysis. Caffeine also potentiated the genotoxicity of known mutagens and enhanced the micronuclei formation (5-fold) in folate-deficient mice. However, caffeine did not increase chromosomal aberrations in in vitro Chinese hamster ovary cell (CHO) and human lymphocyte assays and was not mutagenic in an in vitro CHO/hypoxanthine guanine phosphoribosyltransferase (HGPRT) gene mutation assay, except at cytotoxic concentrations. In addition, caffeine was not clastogenic in an in vivo mouse micronucleus assay. Caffeine was negative in the in vitro bacterial reverse mutation assay (Ames test).
Impairment of Fertility: Caffeine (as caffeine base) administered to male rats at 50 mg/kg/day subcutaneously (0.7 times the daily dose of 360 mg caffeine on a mg/m2 basis) for 4 days prior to mating with untreated females, caused decreased male reproductive performance in addition to causing embryotoxicity. In addition, long-term exposure to high oral doses of caffeine (3 g over 7 weeks) was toxic to rat testes as manifested by spermatogenic cell degeneration.
Developmental Toxicity: In studies performed in adult animals, caffeine (as caffeine base) administered to pregnant mice as sustained release pellets at 50 mg/kg (0.7 times the human daily dose of 360 mg caffeine on a mg/m2 basis), during the period of organogenesis, caused a low incidence of cleft palate and exencephaly in the fetuses.
7.0 Description
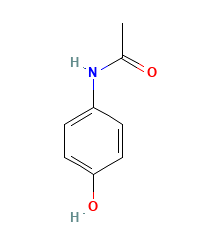
Paracetamol, also called as acetaminophen, is a slightly bitter, white, odorless, crystalline powder. Paracetamol is a non-opiate, non-salicylate analgesic and antipyretic agent.
Molecular Weight: 151.16 g/mol.
Chemical Name: 4'-hydroxyacetanilide.
Molecular Formula: C8H9NO2.
Structural Formula:
Phenylephrine Hydrochloride
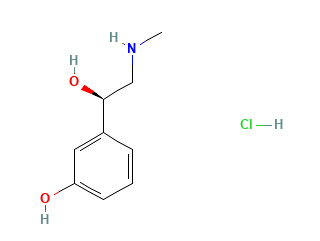
Phenylephrine hydrochloride is an odorless white microcrystalline powder with a bitter taste.
Molecular Weight: 203.66 g/mol.
Molecular Formula: C9H14ClNO2.
Chemical Name: 3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenol; hydrochloride.
Structural Formula:
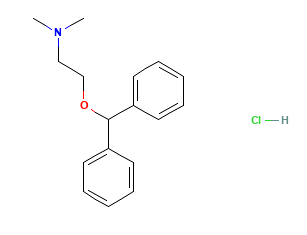
Diphenhydramine Hydrochloride
Diphenhydramine hydrochloride is the hydrochloride salt form of diphenhydramine. Diphenhydramine is first-generation histamine (H1) antagonist with anti-allergic activity. Diphenhydramine hydrochloride appears as white or almost-white crystalline powder.
Molecular Weight: 291.82 g/mol.
Molecular Formula: C17H22ClNO.
Chemical Name: 2-benzhydryloxy-N,N-dimethylethanamine;hydrochloride.
Structural Formula:
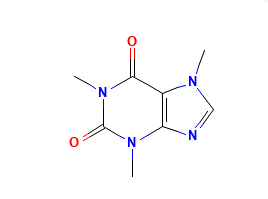
Caffeine
Caffeine is a methylxanthine alkaloid that occurs naturally in seeds, leaves and fruit of several plants. Caffeine acts as a central nervous system (CNS) stimulant. Caffeine appears as odorless white powder or white glistening prismatic crystals.
Molecular Weight: 194.19 g/mol.
Molecular Formula: C8H10N4O2.
Chemical Name: 1,3,7-trimethylpurine-2,6-dione.
Structural Formula:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None known
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
Strip of 10 tablets
8.4 Storage and handing instructions
Store below 30°C. Protect from moisture.
Keep away from the reach of children.
9.0 Patient Counselling Information
Warning: Taking more than daily dose of Paracetamol may cause serious liver damage or allergic reactions.
Administration Instructions to Patients
- Use this medication exactly as prescribed by your doctor. Take this tablet with or after food.
- This medicine is not recommended in children below 12 years of age.
- Pregnant women and lactating mothers should not take this medicine.
- Not to share this medication with other patients even though symptoms are similar. Also, don’t use medication prescribed for other patients.
- This medicine is not advisable in patients with severe liver and/or kidney dysfunction.
- Not to use with any other medicine containing paracetamol (prescription or over-the-counter). Users to ask a doctor or pharmacist, if they are not sure about presence of paracetamol in the drug taken for other illnesses. Also, not to use this medicine with other cough and cold relief products without consulting your doctor.
- This medicine (as it contains diphenhydramine) may cause drowsiness and has an additive effect with alcohol. Patients should be warned not to engage in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc. after taking this medicine.
12.0 Date of revision
13 July 2024
About Leaflet
Please read right through this leaflet before you start using this medicine.
- Keep this leaflet you may need to read it again.
- If you have any questions, or if there is anything you do not understand, ask your pharmacist/doctor.
In this leaflet:
- What MAXTRA® COLD + Tablets do
- Check before you take MAXTRA® COLD + Tablets
- How to take MAXTRA® COLD + Tablets
- Possible side effects
- How to store MAXTRA® COLD + Tablets
- Further information
1. What MAXTRA® COLD + Tablets do
MAXTRA® COLD + Tablets provide effective relief from the major cold and flu symptoms. These symptoms include headache, shivers, aches and pains, blocked nose and painful sinuses, catarrh, sore throat, fatigue and tiredness. This medicine contains four active ingredients. Paracetamol is a painkiller and reduces your temperature when you have a fever. Phenylephrine hydrochloride unblocks your nose and sinuses helping you to breathe more easily. Diphenhydramine, which is an antihistamine that helps relieve coughing, sneezing and runny nose. Caffeine is present as a mild stimulant to relieve fatigue and tiredness.
2. Check before you take MAXTRA® COLD + Tablets
Do not take MAXTRA® COLD + Tablets:
- if you have ever had an allergic reaction to paracetamol, diphenhydramine, phenylephrine hydrochloride, caffeine or any of the other ingredients (listed in Section 6).
- if you have kidney or liver problems, overactive thyroid, diabetes, high BP or heart disease.
- if you have phaeochromocytoma or glaucoma
- if you are taking tricyclic antidepressants (e.g. imipramine or amitriptyline) or if you are taking or have taken within the last 2 weeks’ monoamine oxidase inhibitors (MAOIs) (e.g. moclobemide) prescribed for depression.
- if you are taking beta-blockers (e.g. atenolol)
- If you are using any other medicines containing diphenhydramine, including those used on large areas of the skin
- If you have an overactive thyroid gland.
Do not take anything else containing paracetamol while taking this medicine. Do not take with any other flu, cold or decongestant product.
Take special care with MAXTRA® COLD + Tablets
- Contains paracetamol. Taking too much paracetamol can cause serious harm to your liver.
- This product may cause dizziness. If affected do not drive or operate machinery.
Ask your doctor before you take this medicine:
- if you have a blood vessel disease such as Raynaud’s Phenomenon
- if you have an enlarged prostate
- if you have heart or circulation disease.
- If you have a severe infection as this may increase the risk of metabolic acidosis.
- Signs of metabolic acidosis include:
- deep, rapid, difficult breathing
- feeling sick (nausea), being sick (vomiting)
- loss of appetite
Contact a doctor immediately if you get a combination of these symptoms.
lf you are taking other medicines
Talk to your doctor or pharmacist before taking MAXTRA® COLD + Tablets if you are taking any prescribed medicines; particularly metoclopramide or domperidone (for nausea [feeling sick or vomiting [being sick]); ergotamine and methlysergide (for migraine) or colestyramine (to lower blood cholesterol) or drugs to lower blood pressure; appetite suppressants or stimulants; drugs to treat depression such as tricyclic antidepressants (e.g. amitriptyline) or heart disease (e.g. digoxin) or if you take blood thinning drugs (anticoagulants e.g. warfarin).
Pregnancy and breast feeding
Due to the phenylephrine and caffeine content of this product it should not be used if you are pregnant or breast feeding unless advised by a doctor.
3. How to take MAXTRA® COLD + Tablets
Adults, and the elderly: 1 tablet to be administered 3 to 4 times daily as required.
Do not take more than 4 doses in any 24-hour period.
Do not give to children under 12 years of age.
- Do not take more frequently than every 4 hours.
- Do not take more than the recommended dose.
- Always use the lowest effective dose to relieve your symptoms.
- Do not take for more than 7 days without asking your doctor.
- Avoid too much caffeine in drink like coffee and tea. High caffeine intake can cause difficulty sleeping, shaking and an uncomfortable feeling in the chest.
If you take too much….
Seek immediate medical advice if you take too much of this medicine even if you feel well. This is because too much paracetamol can cause delayed, serious liver damage. If your symptoms persist, see your doctor.
4. Possible side effects
Like all medicines, MAXTRA® COLD + Tablets can have side effects, but not everybody gets them. A small number of people have had side effects.
Stop taking this medicine and tell your doctor immediately if you experience:
- Allergic reactions which may be severe such as skin rash and itching sometimes with swelling of the mouth or face or shortness of breath. Very rare cases of serious skin reactions have been reported.
- Skin rash or peeling, or mouth ulcers.
- Breathing problems. These are more likely if you have experienced them before when taking other painkillers such as ibuprofen or aspirin.
- Unexplained bruising or bleeding.
- Reoccurring fevers or infections.
- Nausea, sudden weight loss, loss of appetite and yellowing of the eyes and skin.
- Visual disturbances. This is rare but is more likely in those with glaucoma.
- Unusually fast pulse rate or a sensation of an unusually fast or irregular heartbeat.
- Difficulty passing water. This is more likely to occur in men with an enlarged prostate gland. These reactions are rare.
The following side effects may occur.
Tell your doctor if you get them.
- Raised blood pressure, headache, dizziness, difficulty sleeping, nervousness, anxiety, diarrhoea or sickness.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: https://www.zuventus.com/drug-safety-reporting
Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. Further information
Active ingredients
Each tablet contains
Paracetamol 500 mg, Phenylephrine Hydrochloride 5 mg, Diphenhydramine Hydrochloride 25 mg and Caffeine 25 mg.
Packs of MAXTRA® COLD + Tablets contain 10 tablets in each blister strip.
This leaflet was last revised in June 2024.