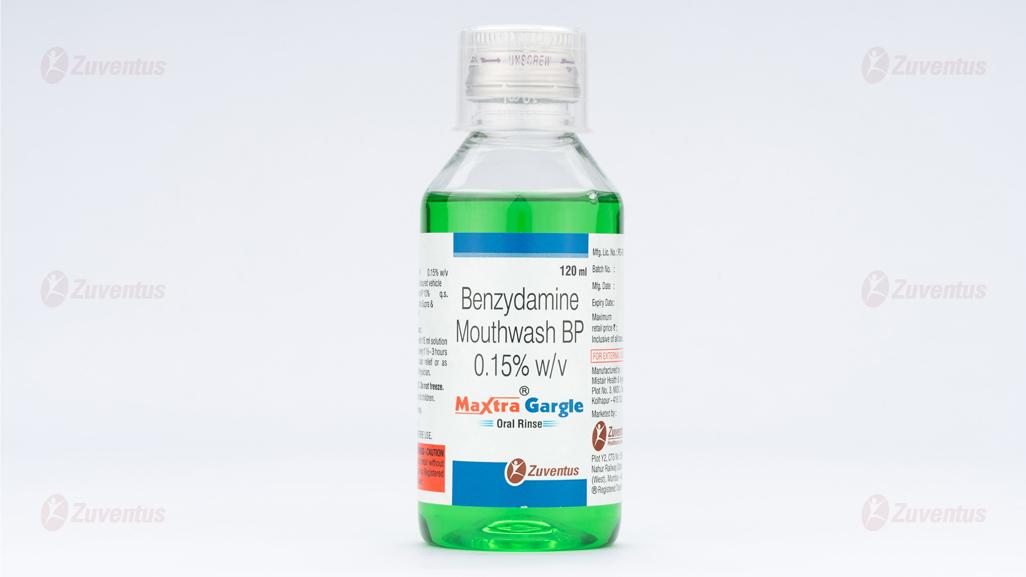
Maxtra Gargle
Therapy Area
Respiratory
1.0 Generic Name
Benzydamine Mouthwash BP 0.15% w/v
2.0 Qualitative and quantitative composition
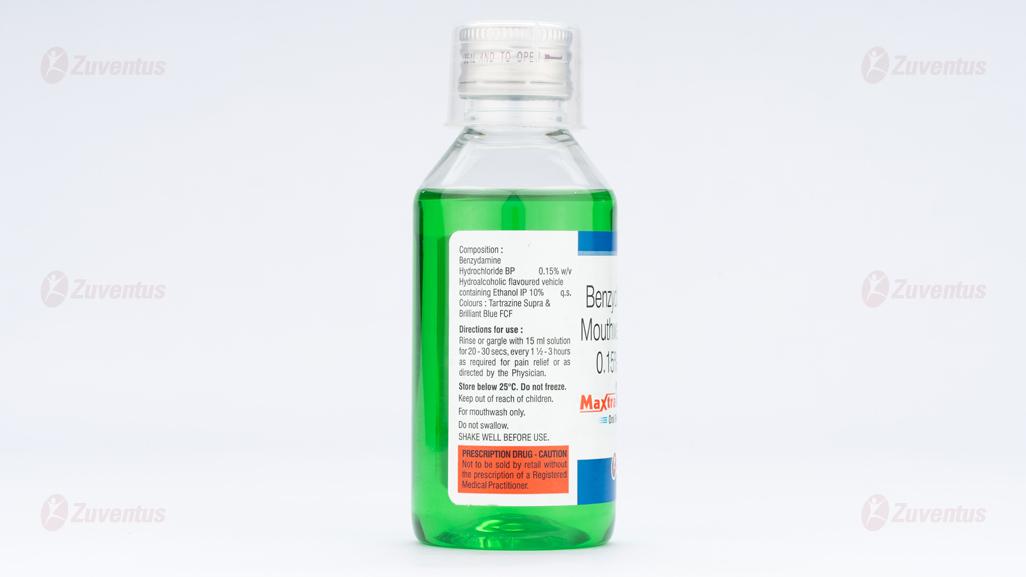
Benzydamine Hydrochloride BP 0.15% w/v
Hydrochloric flavoured vehicle containing Ethanol IP 10% q.s.
3.0 Dosage form and strength
Liquid external (Mouthwash)
0.15% w/v
4.0 Clinical particulars
4.1. Therapeutic indication
Benzydamine Mouthwash is a locally acting analgesic and anti-inflammatory treatment for the relief of painful inflammatory conditions of the mouth and throat including:
- Traumatic Conditions: Pharyngitis following tonsillectomy or the use of a naso-gastric tube;
- Inflammatory Conditions: Pharyngitis, aphthous ulcers and oral ulceration due to radiation therapy;
- Dentistry: For use after dental operations.
4.2. Posology and method of administration
Rinse or gargle with 15 mL solution for 20-30 seconds every 1.5 to 3 hours as required for pain relief. The solution should be expelled from the mouth after use. Benzydamine Mouthwash should generally be used undiluted, but if ‘stinging’ occurs the rinse may be diluted with water.
4.3. Contraindications
Use in patients with a known hypersensitivity to the active ingredient, benzydamine hydrochloride, or to any of the other ingredients.
4.4. Special warnings and precautions for use
- Benzydamine Mouthwash is for oromucosal use only and should not be swallowed.
- Benzydamine use is not advisable in patients with hypersensitivity to acetylsalicylic acid or other NSAIDs.
- Avoid contact with eyes.
- Bronchospasm may be precipitated in patients suffering from or with a previous history of bronchial asthma. Caution should be exercised in these patients.
4.5. Drugs interactions
No interaction studies have been performed.
4.6. Use in special populations
There is no information as regards use of benzydamine in pregnancy. Benzydamine Mouthwash should not be used in pregnancy or lactation unless considered essential by the physician.
4.7. Effects on ability to drive and use machines
Benzydamine Mouthwash has no or negligible influence on the ability to drive and use machines.
4.8. Undesirable effects
The most common side effects are numbness and a stinging feeling in the mouth. The adverse reactions reported are classified by system organ class.
Respiratory, thoracic and mediastinal disorders
Laryngospasm or bronchospasm.
Gastrointestinal disorders
Oral numbness (hypoesthesia) and a stinging feeling in the mouth (oral pain), dryness of mouth
Skin and subcutaneous tissue disorders
Hypersensitivity reactions which may be associated with pruritus, urticaria, photosensitivity reaction and rash, Angioedema
Immune system disorders
Anaphylactic reactions (which can be potentially life-threatening), hypersensitivity reactions
The small amount of alcohol in this medicine will not have any noticeable effects.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com.
Website: http://www.zuventus.co.in/safety.aspx
4.9. Overdose
Intoxication is only expected in case of accidental ingestion of large quantities of benzydamine (> 300 mg).
Very rarely symptoms of overdosing such as excitation, convulsions, sweating, ataxia, tremor and vomiting have been reported after the oral administration of benzydamine dosages much larger than recommended. In the event of acute overdose only symptomatic treatment is possible; the stomach should be emptied by inducing vomiting or by gastric lavage, and the patient carefully observed and given supportive treatment. Adequate hydration must be maintained.
5.0 Pharmacological properties
5.1. Mechanism of Action
The indazole analogue benzydamine has physicochemical properties and pharmacological activities which differ from those of the aspirin-like NSAIDs. Unlike aspirin-like NSAIDs which are acids or metabolised to acids, benzydamine is a weak base. In further contrast, benzydamine is a weak inhibitor of the prostaglandin synthesis. Only at concentration of 1 mM and above benzydamine effectively inhibits cyclooxygenase and lipooxygenase enzyme activity. It mostly exerts its effects through inhibition of the synthesis of pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and Interleukin-1β (IL-1β) without significantly affecting other pro-inflammatory (IL-6 and 8) or anti-inflammatory cytokines (IL-10, IL-1 receptor antagonist). Further mechanisms of action are hypothesised including the inhibition of the oxidative burst of neutrophils as well as membrane stabilisation as demonstrated by the inhibition of granule release from neutrophils and the stabilisation of lysosomes. The local anaesthetic activity of the compound has been related to an interaction with cationic channels.
5.2. Pharmacodynamic properties
Benzydamine specifically acts on the local mechanisms of inflammation such as pain, oedema or granuloma. Benzydamine topically applied demonstrates antiinflammatory activity reducing oedema as well as exudate and granuloma formation. Further, it exhibits analgesic properties if pain is caused by an inflammatory condition and local anaesthetic activity. Hyperthermia, which is indicative of systemic functional involvement, is poorly affected by benzydamine.
5.3. Pharmacokinetic properties
Oral doses of benzydamine are well absorbed and plasma drug concentrations reach a peak fairly rapidly and then decline with a half-life of about 13 hours. Less than 20% of the drug is bound to plasma proteins. Although local drug concentrations are relatively large, the systemic absorption of mouthwash-gargle doses of benzydamine is relatively low compared to oral doses. This low absorption should greatly diminish the potential for any systemic drug side-effects when benzydamine is administered by this route. Benzydamine is metabolized primarily by oxidation, conjugation and dealkylation.
6.0 Nonclinical properties
6.1. Animal Toxicology or Pharmacology
Non-Clinical Data reveal no special hazards for humans based on conventional studies of safety pharmacology, repeated toxicity, genotoxicity, cardiogenic potential, and toxicity to reproduction.
7.0 Description
Benzydamine belongs to the group of nonsteroidal anti-inflammatory drugs.
Molecular Formula: C19H23N3O·HCl
Molecular Weight: 345.87 g/mol
8.0 Pharmaceutical particulars
8.1. Incompatibilities
Not Applicable
8.2. Shelf-life
24 months
8.3. Packaging information
PET clear 120 mL bottle with silver cap and 15 mL measuring cup
8.4. Storage and handing instructions
Store in cool place. Do not refrigerate or freeze.
FOR MOUTHWASH USE ONLY.
DO NOT SWALLOW.
SHAKE WELL BEFORE USE.
FOR EXTERNAL USE ONLY
9.0 Patient Counselling Information
Instruct patients on the following points when administering the drug.
- If a dose of this medication is missed, it is not necessary to make up the missed dose. Skip the missed dose and continue with the next scheduled dose. Do not double doses.
- Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
- Instruct patients to this medicine is for oromucosal use only and should not be swallowed.
- Remind patient to tell about asthmatic condition
- Remind patient to tell about allergic condition to acetylsalicylic acid or to other anti-inflammatory painkillers called NSAIDs
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
- This medicine is available without prescription. However, you still need to use it carefully to get the best results.
- Keep this leaflet. You may need to read it again.
- Ask your doctor, dentist or pharmacist if you need more information or advice.
- You must contact a doctor if your symptoms worsen or do not improve.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Maxtra Gargle Oral Rinse is and what it is used for
2. What you need to know before you use Maxtra Gargle Oral Rinse
3. How to use Maxtra Gargle Oral Rinse
4. Possible side effects
5. How to store Maxtra Gargle Oral Rinse
6. Contents of the pack and other information.
1. What Maxtra Gargle Oral Rinse is and what it is used for
Maxtra Gargle Oral Rinse contains the active substance Benzydamine hydrochloride which is a locally acting analgesic and anti-inflammatory. Maxtra Gargle Oral Rinse used for the relief of painful inflammatory conditions of the mouth and throat including:
- Sore throat following removal of the tonsils
- Mouth ulcers, Sore throat, Mouth ulcers due to radiation therapy
- For use after dental operations, discomfort associated with Dentures or dental work
2. What you need to know before you use Maxtra Gargle Oral Rinse
Do not use Maxtra Gargle Oral Rinse if:
- you are allergic to benzydamine hydrochloride
- you are allergic to any of the other ingredients of Maxtra Gargle Oral Rinse (see section 6).
Warnings and Precautions
Talk to your doctor, pharmacist or nurse before using Maxtra Gargle Oral Rinse:
- If you have a history of asthma
- If you are allergic to acetylsalicylic acid or other non-steroidal anti-inflammatory (NSAID) drugs
If any of these apply to you, talk to your doctor, dentist or pharmacist.
Other medicines and Maxtra Gargle Oral Rinse
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy, breast-feeding and fertility If you are pregnant or breast -feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast -feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
3. How to use Maxtra Gargle Oral Rinse
Important:
Always use Maxtra Gargle Oral Rinse exactly as your Doctor/Pharmacist has told you. You should check with your doctor, dentist or pharmacist if you are not sure.
If you get Maxtra Gargle Oral Rinse in your eyes
Do not use Maxtra Gargle Oral Rinse in or near your eyes.
If any Oral Rinse gets in your eyes, wash them immediately with cold water.
How much Maxtra Gargle Oral Rinse to use
- Adults, adolescents and elderly: The usual dose is 15 ml of solution to be used every 1½ to 3 hours. Use the measuring cup provided to measure the dose.
- Children: This medicine is not suitable for children aged 12 years or under.
How to use the Oral Rinse
The solution should be rinsed around the mouth or used as a gargle for 20 to 30 seconds and then spat out.
This solution should not be swallowed. This solution should not be used for more than 7 days unless your doctor or dentist tells you to.
If your symptoms worsen or do not improve contact your doctor.
If you use more Maxtra Gargle Oral Rinse than you should
If you take too much or you accidentally swallow large quantities of your medicine, contact immediately your doctor or pharmacist for advice.
If you forget to use Maxtra Gargle Oral Rinse
Do not take a double dose to make up for a missed dose. Simply take the next dose as planned. If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines Maxtra Gargle Oral Rinse can cause side effects, although not everybody gets them.
Some side effects could be serious. If you have any of the side effects listed below, seek immediate medical help:
- Serious allergic reaction (anaphylactic shock), signs of which may include difficulty breathing, chest pain or chest tightness, and/or feeling dizzy/faint, severe itching of the skin or raised lumps on the skin, swelling of the face, lips, tongue and/or throat, and which may be potentially life threatening.
Other side effects include the following, if they get serious, please tell your doctor: Uncommon (affects 1 in 1,000 people):
- Numbness and/or stinging in the mouth and/or throat.
Very rare (affects less than 1 in 10,000 people):
- Difficulty breathing and wheezing
- Itching
- Skin rash.
Not known (frequency cannot be estimated from the available data)
- Skin redness or swelling
- Allergic reaction (Hypersensitivity).
If this solution stings your mouth, the next dose can be diluted with some water to reduce this effect.
Some people may react after using the product with a severe allergic reaction experiencing symptoms as described above (a so called anaphylactic reaction) which may be potentially life-threatening. In this case you should seek immediate medical assistance.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Maxtra Gargle Oral Rinse
- Keep out of the sight and reach of children.
- Do not use Maxtra Gargle Oral Rinse after the expiry date on the bottle. The expiry date refers to the last day of that month.
- If you store the product without the original carton, make sure it is kept out of the direct sunlight.
- Medicines should not be disposed of via wastewater or household waste.
- Return any medicine you no longer need to your pharmacist.
6. Contents of the pack and other information
What Maxtra Gargle Oral Rinse contains:
The active substance is benzydamine hydrochloride.
Benzydamine Hydrochloride BP0.15% w/v
Hydrochloric flavoured vehicle containing Ethanol IP 10% q.s.
The other ingredients are tartrazine supra & brilliant blue FCF.
For advice on what to do if you are allergic to any of these ingredients see section 2.
What Maxtra Gargle Oral Rinse looks like
It comes in a PET clear 120 ml bottle with silver cap and 15 ml measuring cup