Maxtra O Nasal Spray
Therapy Area
Respiratory
1.0 Generic Name
Oxymetazoline Hydrochloride Nasal Spray
2.0 Qualitative and quantitative composition
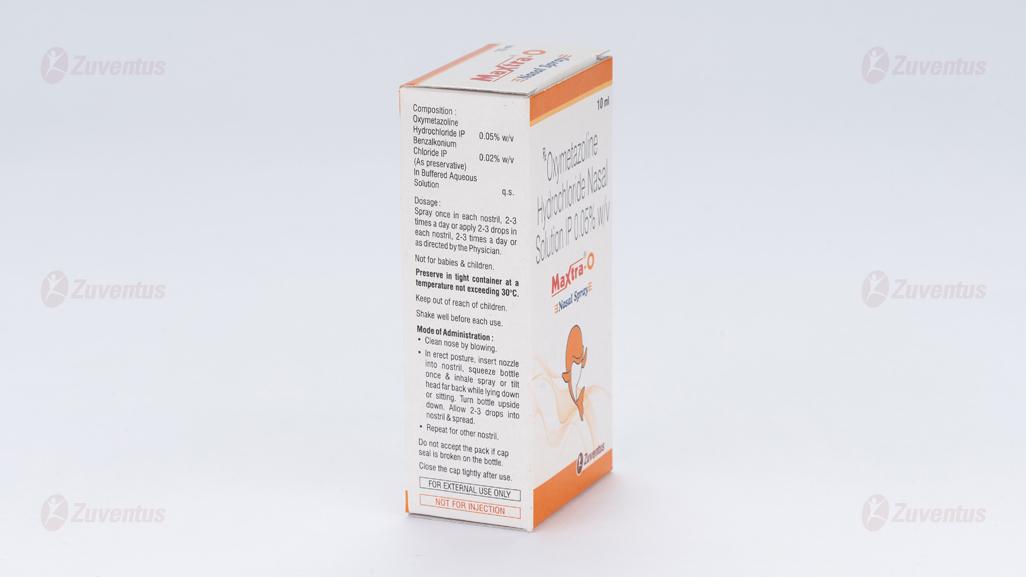
Oxymetazoline Hydrochloride IP 0.05% w/v
Benzalkonium Chloride IP 0.02% w/v
(As preservative)
In buffered aqueous solution q.s
3.0 Dosage form and strength
Nasal Spray
Oxymetazoline (0.05%w/v)
4.0 Clinical particulars
4.1 Therapeutic Indication
For the relief of stuffy, running nose associated with cold and influenza, also post nasal drip, sinusitis.
4.2 Posology and method of administration
Adults and children over 12 years: 1 – 2 sprays up each nostril maximum 2 – 3 times daily. The preparation should not be used for more than 5 – 7 days in a row. Method of administration: Nasal use
4.3 Contraindications
Maxtra-O Nasal Spray should not be used in the following conditions:
In case of hypersensitivity to the active substance or to any of the excipients listed in the formulation.
Patients taking monoamine oxidase inhibitors (MAOIs) or in patients who have taken MAOIs in the previous two weeks.
In patients with narrow-angle glaucoma. Maxtra-O nasal spray should not be used by patients after trans-sphenoidal hypophysectomy.
Children under 12 years of age.
Inflammation of the skin and mucosa of the nasal vestibule and encrustation (rhinitis sicca).
Patients with acute coronary disease or cardiac asthma.
4.4 Special warnings and precautions for use
- Caution should be exercised in case of hypertension, cardiac diseases including angina, hyperthyroidism, diabetes mellitus and prostatic hypertrophy.
- Do not exceed the recommended dose.
- If symptoms worsen or do not improve after 3 days, physician should re-evaluate clinical situation.
- Maxtra-0 Nasal Spray should be used for a maximum of 7 consecutive days to avoid rebound-effect and drug induced rhinitis.
- The preservative (benzalkonium chloride) contained Maxtra-0 Nasal Spray can cause swelling of the nasal mucosa, especially during long-term use. If such a reaction (persistent nasal congestion) is suspected, a product for nasal administration which contains no preservative should be used if possible. If such products for nasal administration are not available without preservative, the use of another dosage form should be considered.
4.5 Drugs interactions
- This product should not be used in combination with MAOIs, or for up to 2 weeks after taking MAOIs as there is a risk of interactions leading to hypertension.
- This product is known to interact with tricyclic antidepressants with a possible increased risk of hypertension and arrhythmias.
- The effects of beta-blockers or other antihypertensive drugs e.g. methyl dopa, bethanidine, debrisoquine and guanethidine may be antagonised.
- Possible additive cardiovascular toxicity may occur when sympathomimetics are given with anti-parkinsonian drugs such as bromocriptine.
4.6 Use in special populations
Pregnancy
For oxymetazoline no clinical data on exposed pregnancies are available. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development.
Breast Feeding
It is unknown whether oxymetazoline hydrochloride is excreted into breast milk. The recommended dose should not be exceeded because overdosing can decrease placental blood flow and reduce milk production. Caution should be exercised during pregnancy and lactation as oxymetazoline may be systemically absorbed
4.7 Effects on ability to drive and use machines
Maxtra-0 Nasal Spray has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Within the system organ classes, adverse reactions are listed under headings of frequency (number of patients expected to experience the reaction), using the following categories, very common (≥1/10); common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100); rare (≥1/10,000 to <1/1,000), very rare (<1/10,000) and not known (cannot be estimated from the available data).


Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Symptoms of Overdose
Symptoms of moderate or severe overdose can be mydriasis, nausea, cyanosis, fever, spasms, tachycardia, cardiac arrhythmia, cardiac arrest, hypertension, oedema of the lungs, dyspnoea, psychic disturbance. The inhibition of functions of the central nervous system such as somnolence, lowering of the body temperature, bradycardia, shock like hypotension, apnoea and loss of consciousness is also possible.
Treatment of overdose
Symptomatic treatment of overdose is required. A nonselective alpha-lytic such as phentolamine may be administered to depress the increased blood pressure, Intubation and artificial respiration may be necessary in serious cases. In the case of moderate or severe inadvertent oral consumption, the administration of activated carbon (adsorbent) and sodium sulphate (laxative) or perhaps gastro-lavage in the case of large amounts should be undertaken. Further treatment is supportive and symptomatic. Vasopressor drugs are contraindicated.
5.0 Pharmacological properties
5.1 Pharmacodynamic properties
Oxymetazoline is a direct-acting sympathomimetic amine. It acts on alpha-adrenergic receptors in the blood vessels of the nasal mucosa producing vasoconstriction and decongestion. Onset of action is within minutes and lasts up to 12 hours.
5.2 Pharmacokinetic properties
Absorption
With local use on the nasal mucosa, there is no clinically relevant absorption of oxymetazoline hydrochloride
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Preclinical data reveal no special hazard for humans based on conventional studies of repeated dose toxicity or toxicity to reproduction. Oxymetazoline Hydrochloride Nasal Spray has not been tested for genotoxicity or carcinogenicity. Preclinical data suggest that benzalkonium chloride can produce a concentration- and time-dependant toxic effect on cilia, including irreversible immobility, and can induce histopathological changes in the nasal mucosa.
7.0 Description
Oxymetazoline is a member of the class of phenols that is 2,4-dimethylphenol which is substituted at positions 3 and 6 by 4,5-dihydro-1H-imidazol-2-ylmethyl and tert-butyl groups, respectively. A direct-acting sympathomimetic with marked alpha-adrenergic activity, it is a vasoconstrictor that is used (generally as the hydrochloride salt) to relieve nasal congestion. It has a role as an alpha-adrenergic agonist, a sympathomimetic agent, a nasal decongestant and a vasoconstrictor agent.
Oxymetazoline
Chemical Name: 6-tert-butyl-3-(4,5-dihydro-1H-imidazol-2-ylmethyl)-2,4-dimethylphenol. Molecular formula: C16H24N2O Molecular weight: 260.37 g/mol Structural formula:

8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf-life
Refer on the pack
8.3 Packaging information
A bottle of 10 ml.
8.4 Storage and handing instructions
Preserve in tight container at a temperature not exceeding 30°C.
Keep out of reach of children.
Do not accept the pack if cap seal is broken on the bottle.
Close the cap tightly after use.
9.0 Details of manufacturer
Zuventus healthcare Ltd.
Plot Y2, CTS No: 358/A2, Near
Nahur Railway Station, Nahur
(West), Mumbai - 400 078, India
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
In this leaflet:
1. What Maxtra-O Nasal Spray is and what it is used for
2. What you need to know before you take Maxtra-O Nasal Spray
3. How to take Maxtra-O Nasal Spray
4. Possible side effects
5. How to store Maxtra-O Nasal Spray
6. Contents of the pack and other information
1. What Maxtra-O Nasal Spray is and what it is used for
Maxtra-O Nasal Spray contains Oxymetazoline Hydrochloride which is a decongestant. It relieves a stuffy, blocked nose by shrinking the blood vessels helping to open the nasal passages. It acts within a few minutes and lasts for up to 12 hours.
The medicine is a solution which is sprayed into the nostrils to relieve the congestion in the nose and sinuses which you get in conditions such as cold, flu, catarrh, sinusitis (inflammation of the passages leading to the nose).
2. Check before you take Maxtra-O Nasal Spray
Do not take Maxtra-O Nasal Spray:
- If you are allergic to oxymetazoline or any of the other ingredients of this medicine
- If you have phaeochromocytoma (a rare tumour of the adrenal glands)
- If you have had an operation to remove your pituitary gland or you have recently had an operation on your nose or sinuses
- If you are already taking medicines to unblock your nose
- If you have inflamed skin or mucous membranes of your nostrils or have scabs in your nose. If your child is under 12 years of age
Talk to your doctor before you take this medicine
- If you have prostate problems
- If you are elderly
- If you have glaucoma (increased pressure in the eyes)
- If you have coronary artery disease, cardiac asthma, or any other heart condition
- If you have high blood pressure
- If you have an over-active thyroid gland or other problems with your thyroid function
- If you have diabetes
- If you have kidney or liver problems
Contact a doctor immediately if you get a combination of these symptoms.
If any of the following occur, this medicine should be stopped:
- Hallucinations
- Restlessness
- Sleep disturbances
If you are taking other medicines
Talk to your doctor or pharmacist before taking Maxtra-O Nasal Spray if you are taking any prescribed medicines; particularly
- Do not use if you are taking or have recently taken Monoamine Oxidase Inhibitors
- Do not use if you are taking beta blockers as their effect may be reduced
- Tricyclic antidepressants (drugs used in the treatment of depression such as amitriptyline and imipramine)
- High blood pressure medicines (such as bethanidine, debrisoquine and guanethidine) as their effect may be reduced
- Parkinson's disease medicine (bromocriptine and rasagiline) treatment may be affected if it is used at the same time
- Digoxin or digitoxin for heart failure, as it can cause irregular heart beat (dysrhythmias) if it is taken at the same time
- Ergotamine or methysergide for migraine relief
- Medicines or supplements to reduce appetite or make you more alert (amphetamine-like psychostimulants)
- Thyroid hormones e.g. levothyroxine
- Oxytocin used to assist birth or breastfeeding.
If you are taking any other medicines, you should see your doctor or pharmacist for advice before using this medicine.
Driving and using machines: No effect on ability to drive or use of machinery has been observed.
Pregnancy and breast feeding
Do not use if you are pregnant. Talk to your doctor or pharmacist before using the nasal spray if you are breastfeeding.
Important information about some of the ingredients in this medicine:
- Contains benzalkonium chloride which may cause irritation or swelling inside the nose, especially if used for a long time.
Tell your doctor if you have any known allergies.
3. How to take Maxtra-O Nasal Spray
For nasal use only

Check if the seal is not broken before first use. If it is, do not give the medicine.
| Age | Dose and how to use | How often to use |
| Adults, the elderly and children aged 12 years and above | See diagram Remove the cap. Hold the container upright, place the tip of the spray nozzle just into each nostril. Hold the other nostril closed. Lightly squeeze the container twice as you breathe in through the nose at the same time. Repeat for the other nostril. Wipe the nasal applicator with a clean, wet tissue and replace the cap immediately after application. | The nasal spray dose may be applied 1-2 sprays up each nostril maximum 2-3 times daily, or used at bedtime to give relief through the night. |
Do not give to children under 12 years.
Do not exceed the stated dose.
Do not use continuously for more than 5-7 days.
This product is not suitable for long-term use.
If symptoms persist, consult your doctor.
If you do not understand these instructions or if you require any further information about your medicine, ask your doctor or pharmacist.
If you use too much:
If you have used more spray than the stated dose, talk to a doctor or pharmacist immediately
4. Possible side effects
Like all medicines, Maxtra-O Nasal Spray can have side effects, but not everybody gets them.
- Allergic reaction
Stop using the nasal spray and contact your doctor or go to your nearest hospital casualty department immediately if you notice:
- skin rashes or itching
- sneezing or stinging or burning of the nose
- swelling of the nose, lips, tongue or throat
- shortness of breath or difficulty in breathing.
Other side effects
- Irritation, dryness or soreness in the nose, mouth or throat
- Eye irritation, dryness, discomfort or redness
- Headache, dizziness
- Weakness, fatigue and trembling
- Anxiety, fear, irritability, nervousness, restless or not sleeping, confused or seeing things
- Nausea (feeling sick), vomiting (being sick), less saliva than usual in your mouth, decreased appetite •Flushing and visual disturbances
- Effects on the heart e.g rapid or irregular heartbeat, palpitations
- Effects on the circulation e.g. cold feet or hands, high blood pressure
- If the spray is used regularly over a long period, it may cause a condition called rebound congestion. This is an unusual swelling of the lining of the nose, leading to itching, sneezing and a blocked nose following the last dose of the spray. Therefore, do not use the spray continuously for more than 5-7 days Prolonged and/or heavy use beyond the recommended use of 7 days, may lead to cardiovascular and/or CNS effects.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Maxtra-O Nasal Spray
Store at a temperature not exceeding 30º C
Keep out of reach of children.
Do not accept the pack if cap seal is broken on the bottle.
Close the cap tightly after use.
6. Contents of the pack and other information
What Maxtra-O Nasal Spray contains
Oxymetazoline Hydrochloride IP 0.05% w/v
Benzalkonium Chloride IP (as preservative) 0.02% w/v
Packing
A bottle of 10 ml
Marketing Authorisation Holder
Zuventus Healthcare Limited
Zuventus House, Plot Y2, CTS No.: 358/A2,
Near Nahur Railway Station,
Nahur (W), Mumbai, 400078 Maharashtra, India
This leaflet was last revised in 07/2024