Maxtra Oral Drops
Therapy Area
Respiratory
1.0 Generic name
Phenylephrine Hydrochloride & Chlorpheniramine Maleate Drops IP (5 mg + 2 mg)
2.0 Qualitative and quantitative composition
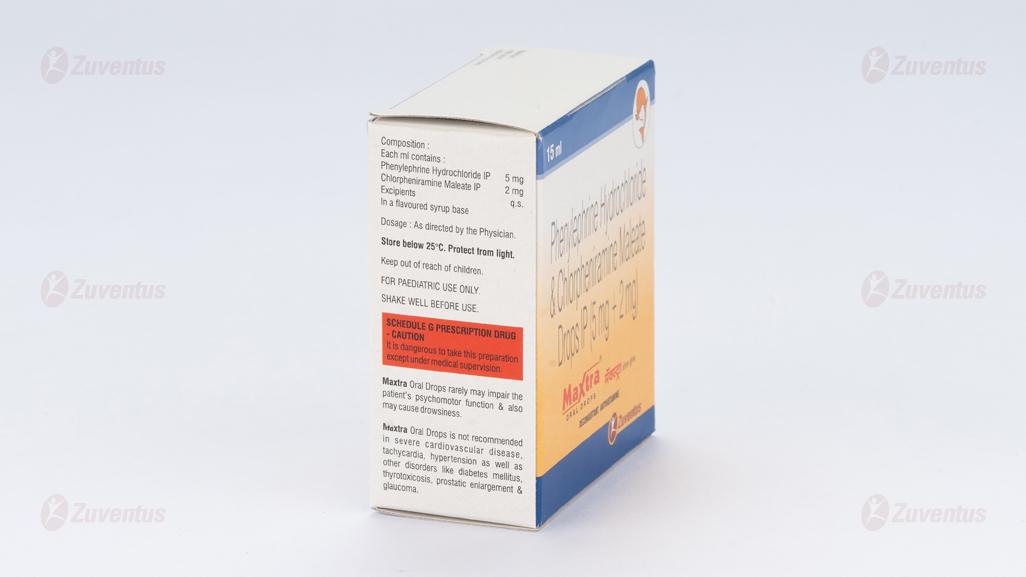
Each ml contains :
Phenylephrine Hydrochloride IP 5 mg
Chlorpheniramine Maleate IP 2 mg
Excipients q.s.
In a flavoured syrup base
3.0 Dosage form and strength
Oral Drops, 5mg/2mg.
4.0 Clinical particulars
4.1 Therapeutic indication
Treatment of cough and cold with nasal congestion.
4.2 Posology and method of administration
Adults and children > 12 years
- 2 ml every 4 to 6 hours
In children > 6 to 12 years
- 1 ml every 4 to 6 hours
In children 4 to 6 years
- 0.5 ml every 4 to 6 hours or as directed by physician.
Maxtra drops should not be used in children below 4 years of age.
Do not give continuously for more than 5 days without medical advice.
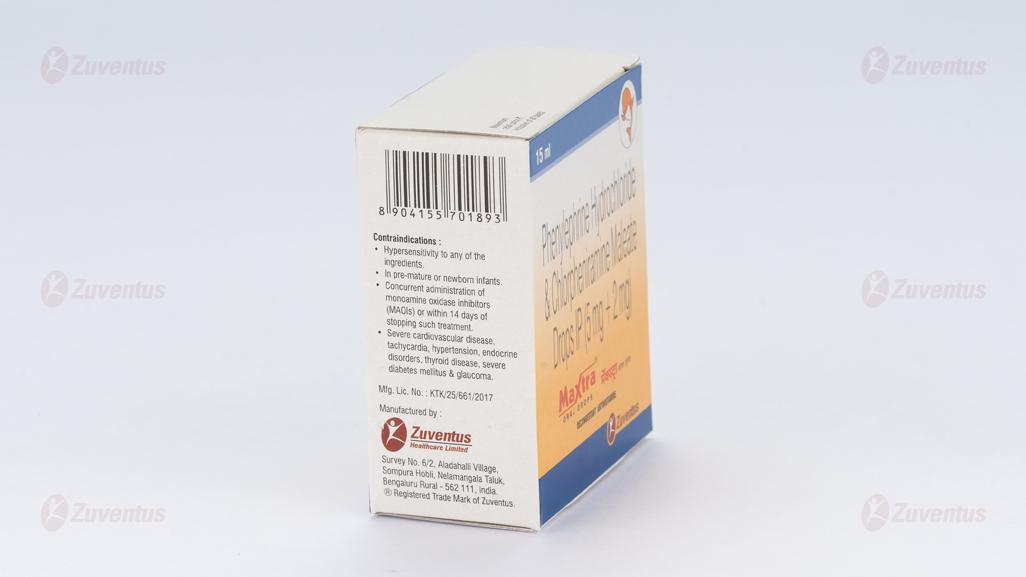
4.3 Contraindications
- Patients who are hypersensitive to antihistamines or to any of the Oral Drops ingredients.
- Concurrent administration of monoamine oxidase inhibitors (MAOIs) or within 14 days of stopping such treatment.
- Acute asthma.
- Premature infants or neonates because of their increased susceptibility to the antimuscarinic effects.
- Concomitant use of other sympathomimetic decongestants.
- Phaeochromocytoma.
- Severe cardiovascular disease, tachycardia, hypertension, endocrine disorders, thyroid disease, thyrotoxicosis, prostatic enlargement, severe diabetes mellitus, glaucoma & hepatic or severe renal impairment.
4.4 Special warnings and precautions for use
Maxtra drops should not be used in children below 4 years of age.
This medicine should be given with caution to patients with epilepsy, severe cardiovascular disorders, liver disorders, glaucoma, urinary retention, pyloroduodenal obstruction, asthma, bronchitis, bronchiectasis, thyrotoxicosis and severe hypertension.
Special care should be taken when using chlorpheniramine maleate in children, as they are more prone to developing neurological anticholinergic effects. This product should not be used in patients taking other sympathomimetics (such as decongestants).
Patients suffering from chronic cough or asthma should consult a physician before taking this product.
Patients should stop using the product and consult a health care professional if cough lasts for more than 5 days or comes back, or is accompanied by a fever, rash or persistent headache.
Warning:
May cause drowsiness.
Keep all medicines out of the reach of children.
Although most antihistamines should be avoided by patients with porphyria, chlorpheniramine maleate has been used and is thought to be safe.
Special label warnings
Do not take with other flu, cold or decongestant products.
Do not take with a cough suppressant.
Do not exceed the recommended dose.
4.5 Drugs interactions
Chlorpheniramine Maleate
This medicine may enhance the sedative effects of hypnotics, anxiolytics, sedatives, opioid analgesics and neuroleptics.
The antimuscarinic effects of chlorpheniramine are enhanced by other antimuscarinic drugs and both antimuscarinic and sedative effects are enhanced by MAOIs and tricyclic antidepressants.
Metabolism of phenytoin may be inhibited by chlorpheniramine with the possible development of phenytoin toxicity.
Phenylephrine hydrochloride
Phenylephrine should be used with caution in combination with the following drugs as interactions have been reported :
| Monoamine oxidase inhibitors (including moclobemide) | Hypertensive interactions occur between sympathomimetic amines such as phenylephrine and monoamine oxidase inhibitors (see contraindications). |
| Sympathomimetic amines | Concomitant use of phenylephrine with other sympathomimetic amines can increase the risk of cardiovascular side effects. |
| Beta-blockers and other antihypertensives | Phenylephrine may reduce the efficacy of beta-blocking drugs and antihypertensive drugs. The risk of hypertension and other cardiovascular side effects may be increased. |
| Tricyclic antidepressants (e.g. amitriptyline) | May increase the risk of cardiovascular side effects with phenylephrine. |
| Ergot alkaloids (ergotamine and methylsergide) | ncreased risk of ergotism. |
| Digoxin and cardiac glycosides | Increase the risk of irregular heartbeat or heart attack. |
If urine is collected within 24 hours of a dose of this product, a metabolite may cause a colour interference with laboratory determinations of 5 hydroxy-indole-acetic acid (5-HIAA) and vanilly-mandelic acid (VMA).
4.6 Use in special populations
Pregnancy
Information regarding the use of Maxtra Oral Drops during pregnancy is not available. Please consult your doctor.
Lactation
Information regarding the use of Maxtra Oral Drops during breastfeeding is not available. Please consult your doctor.
4.7 Effects on ability to drive and use machines
Maxtra Oral Drops rarely may impair the patients psychomotor function & also may cause drowsiness. Do not drive if you experience any such symptoms that affect your ability to concentrate and react.
4.8 Undesirable effects
Chlorpheniramine Maleate
The product may cause drowsiness, which may progress to deep sleep, headache, dizziness, psychomotor impairment, inability to concentrate, lassitude, irritability and antimuscarinic effects such as urinary retention, dry mouth and blurred vision.
Gastrointestinal disturbances may occur including abdominal pain, dyspepsia and anorexia.
Paradoxical CNS stimulation may occur especially in children or after high doses.
Skin rashes including exfoliative dermatitis and photosensitivity reactions and hypersensitivity reactions including urticaria may occur.
Other side effects include convulsions, sweating, myalgia, paraesthesia, tinnitus, palpitations, tachycardia, arrhythmias, chest pain, haemolytic anaemia and other blood dyscrasias, extrapyramidal effects, tremor, liver dysfunction, including hepatitis and jaundice, sleep disturbances, including nightmares, depression, hypotension, hair loss, thickening of bronchial secretions and confusional psychosis in the elderly.
Phenylephrine hydrochloride
The following adverse events have been observed in clinical trials with phenylephrine and may therefore represent the most commonly occurring adverse events.
| Body System | Undesirable effect |
| Psychiatric disorders | Nervousness, irritability, restlessness, and excitability |
| Nervous system disorders | Headache, dizziness, insomnia |
| Cardiac disorders | Increased blood pressure |
| Gastrointestinal disorders | Nausea, Vomiting, diarrhoea |
Adverse reactions identified during post-marketing use are listed below. The frequency of these reactions is unknown but likely to be rare.
| Eye disorders | Mydriasis, acute angle closure glaucoma, most likely to occur in those with closed angle glaucoma. |
| Cardiac disorders | Tachycardia, palpitations |
| Skin and subcutaneous disorders | Allergic reactions (e.g. rash, urticaria, allergic dermatitis). Hypersensitivity reactions - including that crosssensitivity may occur with other sympathomimetics. |
| Renal and urinary disorders | Dysuria, urinary retention. This is most likely to occur in those with bladder outlet obstruction. |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
Website : http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Treatment with activated charcoal should be considered or gastric lavage and aspiration should be performed. Treatment is most effective if given within an hour of ingestion. Hypotension and arrhythmias should be treated vigorously. Symptomatic and supportive measures should be undertaken, particularly with regard to cardiovascular and respiratory systems.
5.0 Pharmacological properties
5.1 Mechanism of action/ Pharmacodynamic properties
Chlorpheniramine Maleate
Chlorpheniramine antagonises competitively the effects of histamine on H1-receptors and also has weak antimuscarinic and moderate anti-serotonin and local anaesthetic actions. It penetrates the brain and causes stimulation or sedation in animals.
Phenylephrine hydrochloride
Phenylephrine is a sympathomimetic agent with mainly direct effects on adrenergic receptors. It has predominantly alpha adrenergic activity and is without stimulating effects on the central nervous system. The sympathomimetic effect of phenylephrine produces vasoconstriction which in turn relieves nasal congestion.
5.2 Pharmacokinetic properties
Chlorpheniramine Maleate
Chlorpheniramine maleate is almost completely absorbed after administration by mouth, peak plasma concentrations occurring at about 2.5 to 6 hours. The drug is widely distributed including passage into the CNS, with a volume of distribution of between 1 and 10 L/kg. About 70% of chlorpheniramine in the circulation is protein-bound. Chlorpheniramine undergoes some first pass metabolism and enterohepatic recycling. Chlorpheniramine is extensively metabolised, principally to inactive desmethylated metabolites which are excreted primarily in the urine, together with about 35% unchanged drug. Only trace amounts are excreted in the faeces. The mean elimination half-life has been reported to be about 30 hours, with mean values ranging from 2 to 43 hours.
Phenylephrine hydrochloride
Phenylephrine hydrochloride is irregularly absorbed from the gastrointestinal tract and undergoes first-pass metabolism by monoamine oxidase in the gut and liver; orally administered phenylephrine thus has reduced bioavailability. It is excreted in the urine almost entirely as the sulphate conjugate.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
The antihistaminic potency of chlorpheniramine is confined mainly to its (+)-isomer. The racemate is similarly or slightly more toxic because of the contribution of (-)-isomer. The toxicity may therefore be non-specific, perhaps attributable to local anaesthetic action and the toxic effects (excitation/sedation, coma, convulsions and death) resemble those of other classic H1 antihistamines. Toxic doses may cause hypotension attributable to myocardial depression, an effect which is clearer with the (-)-isomer.
The experimental data on the carcinogenicity and mutagenicity of chlorpheniramine indicate lack of these adverse effects, but the racemate and the (+)-isomer have shown some embryotoxicity in fertility tests.
Effective antihistaminic concentrations of chlorpheniramine in vitro are about 1-10µg/L and oral doses of 0.2-1 mg/kg antagonise histamine-induced bronchospasm in guinea pigs.
7.0 Description
Maxtra Oral Drops contains phenylephrine hydrochloride (a nasal decongestant) and chlorpheniramine maleate (an antihistamine). Each 1 ml Oral Drops contains: Phenylephrine Hydrochloride, 5 mg; Chlorpheniramine Maleate, 2 mg; and Excipients (q.s.)
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
A Bottle of 15 ml.
8.4 Storage and handing instructions
Store below 25°C. Protect from light.
Keep out of reach of children.
9.0 Patient Counselling Information
- Maxtra Oral Drops should not be used in children below 4 years of age.
- Maxtra Oral Drops helps relieve symptoms of cold and flu such as watery eyes, runny nose, sneezing, and itching of the nose or throat.
- Take it as per the dose and duration prescribed by your doctor.
- It may cause dizziness and sleepiness. Don't drive or do anything that requires mental focus until you know how it affects you.
- Avoid consuming alcohol when taking Maxtra Oral Drops as it may cause excessive drowsiness.
- Inform your doctor if you are pregnant or planning to conceive or breastfeeding.
12.0 Date of revision
06 June 2024
About Leaflet
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What MAXTRA® Oral Drops is and what it is used for
- What you need to know before you are given MAXTRA® Oral Drops
- How MAXTRA® Oral Drops is given
- Possible side effects
- How to store MAXTRA® Oral Drops
- Contents of the pack and other information
1. What MAXTRA® is and what it is used for
MAXTRA® is an Oral Drops that contains: Chlorphenamine Maleate, which belongs to a group of medicines called antihistamines, which act to relieve the symptoms of allergic reactions.
Phenylephrine hydrochloride is a decongestant which unblocks your nose and sinuses helping you breathe more easily. MAXTRA® is used to treat cough and cold with nasal congestion.
Maxtra® drops should not be used in children below 4 years of age.
2. What you need to know before you take MAXTRA®
Do not take MAXTRA® Oral Drops:
- If your child is having an asthma attack
- If your child is allergic to any of the ingredients, or any other antihistamines (your child may have had a rash, difficulty breathing, swollen lips or face after taking them)
- If you have kidney or liver problems, overactive thyroid, diabetes, high blood pressure or heart or circulation disease
- If you have phaeochromocytoma or glaucoma
- If you are taking tricyclic antidepressants (e.g. Imipramine or amitriptyline)
- If you are taking beta blockers.
Do not take with any other flu, cold or decongestant product.
Other important information
Pregnancy and breastfeeding: Not applicable.
Other medicines and MAXTRA®
Chlorpheniramine Maleate
Before you give this medicine, make sure that you tell your pharmacist about ANY other medicines you might be giving your child at the same time, particularly the following:
- Other antihistamines
- Strong painkillers
- Medicines for mental problems
- Phenytoin (for epilepsy)
- Atropine
Phenylephrine Hydrochloride
Talk to your doctor or pharmacist before taking this medicine if you are taking any prescribed medicines; particularly
- Metoclopramide or domperidone (for nausea [feeling sick] or vomiting [being sick])
- Ergotamine or methylsergide (for migraine)
- Drugs to lower blood pressure
- Appetite suppressants or stimulants
- Heart disease (e.g. Digoxin)
- Blood thinning drugs (anticoagulants e.g. Warfarin).
If you are unsure about interactions with any other medicines, talk to your pharmacist or doctor. This includes medicines prescribed by your doctor and medicine you have bought for your child, including herbal and homeopathic remedies.
Warnings and precautions
Talk to your doctor or pharmacist before taking MAXTRA®:
- If your child has epilepsy, heart or circulatory disease, liver problems
- If your child has high blood pressure or glaucoma
- If your child has asthma, bronchitis or bronchiectasis
- If your child has an overactive thyroid
- If your child has difficulty passing urine
- If your child has an obstruction in their intestine
- If your child has a rare blood disease called porphyria
- If you have a blood vessel disease such as Raynaud’s Phenomenon
- If you have liver or kidney problems.
- If you have a severe infection as this may increase the risk of metabolic acidosis.
Signs of metabolic acidosis include:
- Deep, rapid, difficult breathing
- Feeling sick (nausea), being sick (vomiting)
- Loss of appetite
Contact a doctor immediately if you get a combination of these symptoms
Driving and using machines
Not applicable.
3. How to take MAXTRA® Oral Drops
Check the seal is not broken before first use. If it is, do not give the medicine. Give this medicine to your child to swallow Do not give more than the amount recommended.
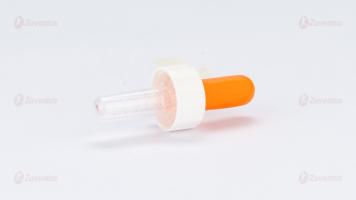
A 1 ml dropper with markings is provided with the solution to administer medication accurately to children. The markings on the dropper allow caregivers to measure precise doses, ensuring the child receives the correct amount of medication. This helps in preventing underdosing or overdosing, which is particularly important for maintaining the efficacy and safety of the treatment in pediatric patients.
The recommended dosage of MAXTRA® in
In children 4 to 6 years: 0.5 ml every 4 to 6 hours or as directed by physician.
In children > 6 to 12 years: 1 ml every 4 to 6 hours
Adults and children > 12 years: 2 ml every 4 to 6 hours
Maxtra® drops should not be used in children below 4 years of age. If symptoms do not go away within 5 days talk to your pharmacist or doctor.
If you take more MAXTRA® than you should
If you have too much of this medicine, talk to your doctor straight away. Take your medicine and this leaflet with you.
4. Possible side effects
Chlorpheniramine Maleate
Most people will not have problems, but some may get some of these.
- Drowsiness (which may make your child fall asleep)
- Dizziness, blurred vision, headaches, fits
- Dry mouth, difficulty in passing urine, sweating
- Skin rash, sensitivity to sunlight, other allergic reactions
- Indigestion, stomach pain, loss of appetite
- Tremors, muscle pain or weakness, impaired movement or co-ordination, pins and needles
- Change in heart rate, palpitations, low blood pressure, ringing in the ears, hair loss
- Blood problems such as anaemia, weariness
- Sleep disturbance
- Liver problems (which may cause yellowing of the skin or eyes)
- Chest pain
- Cough, phlegm on the chest – these may be caused by thickened bronchial secretions (mucous) in your lungs
- Difficulty concentrating, irritability, depression
- Hyperactivity
Phenylephrine Hydrochloride
Stop taking this medicine and tell your doctor immediately if you experience:
- Allergic reactions which may be severe such as skin rash and itching sometimes with swelling of the mouth or face or shortness of breath.
- Skin rash or peeling, or mouth ulcers. Very rare cases of serious skin reactions have been reported.
- Unexplained bruising or bleeding.
- Re-occurring fevers or infections.
- Nausea, sudden weight loss, loss of appetite and yellowing of the eyes and skin.
- Visual disturbances. This is rare but is more likely in those with glaucoma.
- Unusually fast pulse rate or a sensation of an unusually fast or irregular heartbeat.
The following side effects may occur. Tell your doctor if you get them:
- Raised blood pressure, headache, dizziness, difficulty sleeping, nervousness, anxiety, diarrhoea or sickness.
Very young children may be more likely to get some of these side effects.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store MAXTRA® Oral Drops
Do not store above 25°C.
Store in the original package.
Keep this medicine in a safe place out of the sight and reach of children, preferably in a locked cupboard.
Use by the date on the end flap of the carton or on the foil edge.
Do not throw away medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
A Bottle of 15 ml.
What MAXTRA® Oral Drops contains
Each ml contains:
Phenylephrine Hydrochloride IP 5 mg
Chlorpheniramine Maleate IP 2 mg
Excipients q.s.
In a flavoured syrup base
Manufacturer
Zuventus healthcare Ltd.
Plot Y2, CTS No: 358/A2, Near Nahur Railway
Station, Nahur (West), Mumbai - 400 078, India.
At: A-22, M.I.D.C., Additional Ambernath,
District Thane - 421 506.
Registered Trade Mark of Zuventus.
Date of revision
06 June 2024