Maxtra-P DS Syrup
Therapy Area
Respiratory
1.0 Generic Name
Paracetamol, Phenylephrine Hydrochloride & Chlorpheniramine Maleate Syrup
2.0 Qualitative and quantitative composition
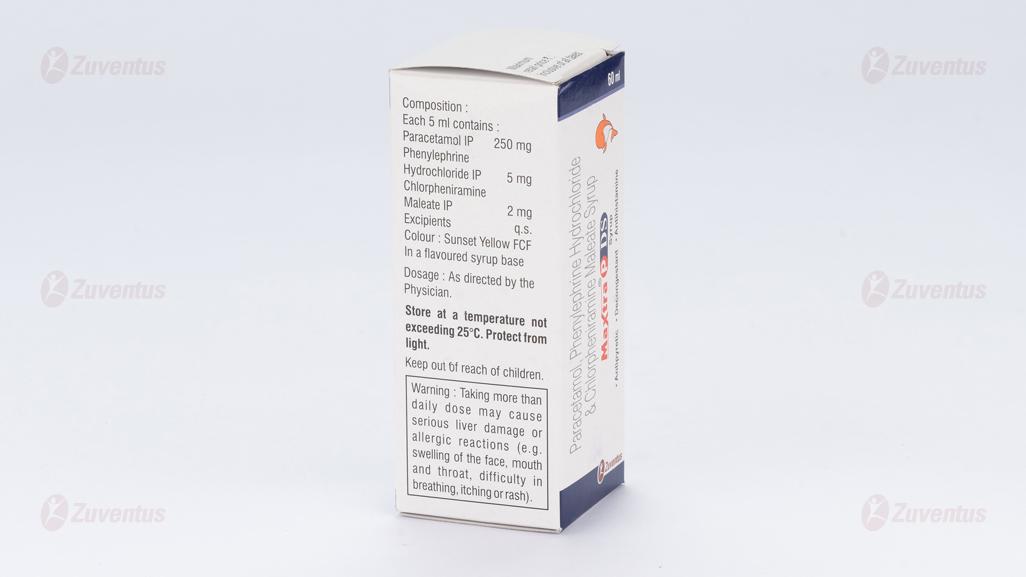
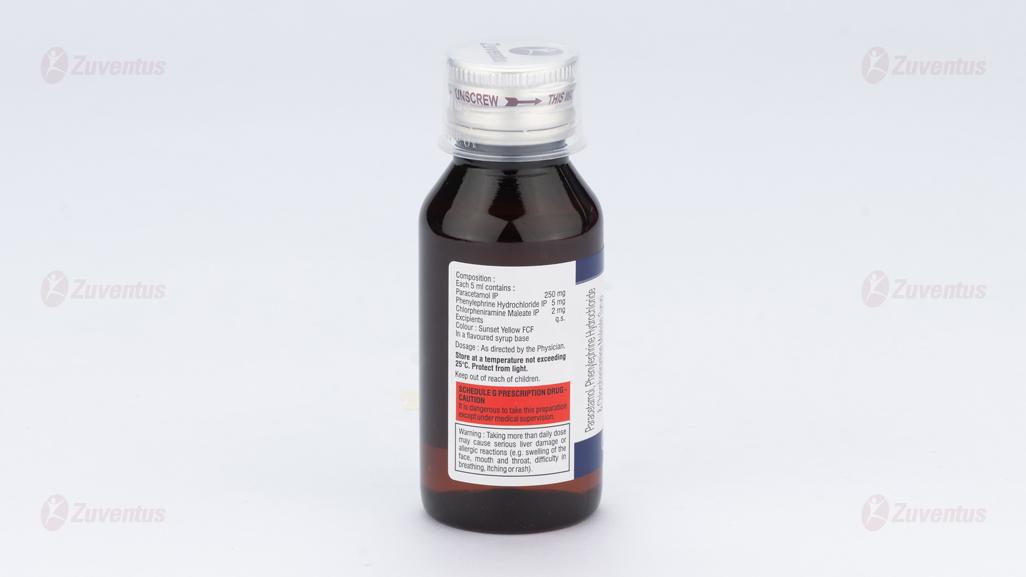
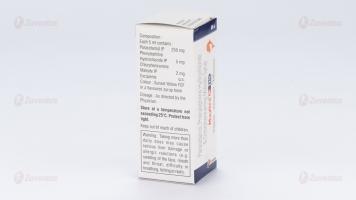
Each 5 ml contains :
Paracetamol IP 250 mg
Phenylephrine Hydrochloride IP 5 mg
Chlorpheniramine Maleate IP 2 mg
Excipients q.s.
Colour : Sunset Yellow FCF
In a flavoured syrup base
3.0 Dosage form and strength
Syrup, Paracetamol 250 mg, Phenylephrine 5 mg, Chlorpheniramine 2 mg
4.0 Clinical particulars
4.1 Therapeutic Indication
For the treatment of common cold and cough associated with fever
4.2 Posology and method of administration
2 to 5 years of age: 2.5 ml every 4 to 6 hours
6 to 11 years of age: 5 ml every 4 to 6 hours
≥12 years of age: 10 ml every 4 to 6 hours
Or as directed by physician
Method of administration: Oral
4.3 Contraindications
• Hypersensitivity to the active substances or to any of the excipients
• Concomitant use of other sympathomimetic decongestants
• Pheochromocytoma
• Closed angle glaucoma
• An enlargement of the prostate gland
• Hypertensive patients or those who are taking, or have taken in the last two weeks, monoamine oxidase inhibitors, tricyclic antidepressants or beta-blockers
• Hepatic or renal impairment, diabetes, hyperthyroidism and cardiovascular disease
4.4 Special warnings and precautions for use
• Use with caution in patients with Raynaud's phenomenon or diabetes mellitus.
• This medicine should be given with caution to patients with epilepsy, severe cardiovascular disorders, liver disorders, glaucoma, urinary retention, prostatic enlargement, pyloroduodenal obstruction, asthma, bronchitis, bronchiectasis, thyrotoxicosis and severe hypertension.
• Care is advised in the administration of paracetamol to patients with severe renal or severe hepatic impairment. The hazard of overdose is greater in those with non-cirrhotic alcoholic liver disease. Patients should be advised not to take other paracetamol-containing products concurrently.
• Special care should be taken when using chlorpheniramine maleate in children and the elderly as they are more prone to developing neurological anticholinergic effects.
• Taking more than daily dose may cause serious liver damage or allergic reactions (eg. Swelling of the face, mouth and throat, difficulty in breathing, itching or rash) Paracetamol, Phenylephrine Hydrochloride & Chlorpheniramine Maleate Syrup
4.5 Drugs interactions
• The anticoagulant effect of warfarin and other coumarins may be enhanced by prolonged regular use of paracetamol with increased risk of bleeding; occasional doses have no significant effect.
• The hepato-toxicity of paracetamol may be potentiated by excessive intake of alcohol.
• The speed of absorption of paracetamol may be increased by metoclopramide or domperidone and absorption reduced by colestyramine.
• Phenylephrine may adversely interact with other sympathomimetics, vasodilators and beta blockers.
• Sympathomimetic-containing products should be used with great care in patients receiving phenothiazines or tricylic antidepressants.
• Sympathomimetic-containing products should be used with caution in patients receiving digitalis, beta-adrenergic blockers, guanethidine, reserpine, methyldopa or anti-hypertensive agents.
• Concurrent use with halogenated anaesthetic agents such as chloroform, cyclopropane, halothane, enflurane or isoflurane may provoke or worsen ventricular arrhythmias.
• This medicine may enhance the sedative effects of alcohol, hypnotics, anxiolytics, sedatives, opioid analgesics and neuroleptics.
• The antimuscarinic effects of chlorpheniramine are enhanced by other antimuscarinic drugs and both antimuscarinic and sedative effects are enhanced by monoamine oxidase inhibitors and tricyclic antidepressant.
4.6 Use in special populations
Pregnancy and lactation There are no adequate controlled studies of chlorpheniramine in pregnant women, Phenylephrine may reduce placental perfusion and this medicine should therefore not be used during pregnancy. Chlorpheniramine may be secreted in breast milk and its use is not recommended in nursing mothers because of the risk of adverse effects, such as unusual excitement or irritability in infants. Chlorpheniramine may also inhibit lactation.
4.7 Effects on ability to drive and use machines
Chlorpheniramine may cause blurred vision, dizzines s, drowsiness and interfere with human performance and therefore may seriously influence the ability to drive and operate machinery.
4.8 Undesirable effects
• Adverse effects of paracetamol are rare but hypersensitivity including skin rashes and Fixed drug eruption (FDE) may occur. There have been reports of blood dyscrasias including thrombocytopenia and agranulocytosis, but these were not necessarily causally related to paracetamol.
• Phenylephrine hydrochloride may elevate blood pressure with headache, vomiting and rarely palpitations, tachycardia or reflex bradycardia, tingling and coolness of the skin. There have been rare reports of allergic reactions.
• Chlorpheniramine may cause drowsiness, which may progress to deep sleep, headache, dizziness, psychomotor impairment, inability to concentrate,lassitude, irritability and antimuscarinic effects such as urinary retention, dry mouth and blurred vision. Gastrointestinal disturbances may occur including abdominal pain, dyspepsia and anorexia. Paradoxical CNS stimulation may occur especially in children or after high doses. Skin rashes including exfoliative dermatitis and photosensitivity reactions and hypersensitivity reactions including urticaria may occur. Other side effects include convulsions, sweating, myalgia, paraesthesia, tinnitus, palpitations, tachycardia, arrhythmias, chest pain, haemolytic anaemia and other blood dyscrasias, extrapyramidal effects, tremor, liver dysfunction, including hepatitis and jaundice, sleep disturbances, including nightmares, depression, hypotension, hair loss, thickening of bronchial secretions and confusional psychosis in the elderly.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Initiate general symptomatic and supportive measures in all cases of over dosages where necessary
5.0 Pharmacological properties
5.1 Mechanism of Action
Paracetamol : Paracetamol has both analgesic and antipyretic activity which is believed to be mediated principally through its inhibition of prostaglandin synthesis within the central nervous system. Phenylephrine : Phenylephrine hydrochloride is an α-1 adrenergic receptor agonist. Chlorpheniramine : Chlorpheniramine antagonises competitively the effects of histamine on H1-receptors and also has weak antimuscarinic and moderate antiserotonin and local anaesthetic actions.
5.2 Pharmacodynamic properties
Paracetamol provides the analgesic and antipyretic actions. Phenylephrine is a post-synaptic α-receptor agonist with low cardioselective β-receptor affinity and minimal central stimulant activity. It is a recognised decongestant and acts by vasoconstriction to reduce oedema and nasal swelling. Chlorpheniramine is an antihistamine drug (H1 receptor antagonist) that also possesses anticholinergic and sedative activity. It prevents released histamine from dilating capillaries and causing edema of the respiratory mucosa
5.3 Pharmacokinetic properties
Paracetamol : Paracetamol is rapidly absorbed from the gastro-intestinal tract with peak plasma concentrations occurring between 10 and 120 minutes after oral administration. It is metabolised in the liver and excreted in the urine mainly as the glucuronide and sulphate conjugates. Less than 5% is excreted as unchanged paracetamol. The elimination half-life varies from about 1 to 4 hours. Phenylephrine : Phenylephrine hydrochloride is irregularly absorbed from the gastrointestinal tract and undergoes first-pass metabolism by monoamine oxidase in the gut and liver; orally administered phenylephrine thus has reduced bioavailability. It is excreted in the urine almost entirely as the sulphate conjugate. Chlorpheniramine : Chlorphenamine is well absorbed from the gastro-intestinal tract, following oral administration. The effects develop within 30 minutes, are maximal within 1 to 2 hours and last 4 to 6 hours. The plasma half-life has been estimated to be 12 to 15 hours. Chlorphenamine is metabolised to the monodesmethyl and didesmethyl derivatives. About 22% of an oral dose is excreted unchanged in the urine.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
The antihistaminic potency of chlorpheniramine is confined mainly to its (+)-isomer. The racemate is similarly or slightly more toxic because of the contribution of (-)-isomer. The toxicity may therefore be non- specific, perhaps attributable to local anaesthetic action and the toxic effects (excitation/sedation, coma, convulsions and death) resemble those of other classic H1antihistamines. Toxic doses may cause hypotension attributable to myocardial depression, an effect which is clearer with the (-)-isomer
The experimental data on the carcinogenicity and mutagenicity of chlorpheniramine indicate lack of these adverse effects, but the racemate and the (+)-isomer have shown some embryotoxicity in fertility tests.
Effective antihistaminic concentrations of chlorpheniramine in vitro are about 1-10µg/L and oral doses of 0.2-1 mg/kg antagonise histamine- induced bronchospasm in guinea pigs.
Preclinical safety data of paracetamol and phenylephrine : None stated
7.0 Description
Maxtra-P DS syrup is a combination of analgesic/antipyretic paracetamol, decongestants phenylephrine hydrochloride and anti-allergic chlorpheniramine maleate.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
A bottle of 60 ml.
8.4 Storage and handing instructions
Store at a temperature not exceeding 25°C. Protect from light.
Keep out of reach of children.
9.0 Patient Counselling Information
• Do not take this medicine, if you are allergic to Paracetamol, Phenylephrine Hydrochloride, Chlorpheniramine Maleate
• Signs of an allergic reaction include a rash, itching or shortness of breath.
• Chlorpheniramine may cause blurred vision, dizziness, drowsiness and interfere with human performance and therefore may seriously influence the ability to drive and operate machinery.
• Advice patient to talk to doctor or pharmacist in case of any side effects. This includes any possible side effects not listed in this leaflet.
• Advice patient to talk to doctor or pharmacist in case of any further questions.
About
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
In this leaflet:
- What Maxtra-P DS Syrup is and what is it used for
- What you need to know before you take Maxtra-P DS Syrup
- How to take Maxtra-P DS Syrup
- Possible side effects
- How to store Maxtra-P DS Syrup
- Contents of the pack and other information
1. What Maxtra-P DS Syrup is and what it is used for
Maxtra-P DS Syrup contains a combination of ingredients which are effective in relieving the symptoms associated with colds and flu, including relief of aches and pains, sore throats, headache, nasal congestion and lowering of temperature.
Paracetamol is a painkiller and reduces your temperature when you have a fever.
Phenylephrine hydrochloride is a decongestant which unblocks your nose and sinuses helping you breathe more easily.
This medicine contains Chlorphenamine Maleate, which belongs to a group of medicines called antihistamines, which act to relieve the symptoms of allergic reactions.
2. What you need to know before you take Maxtra-P DS Syrup
- Do not take Maxtra-P DS Syrup:
- If you have ever had an allergic reaction to any of the ingredients
- If you have kidney or liver problems, overactive thyroid, diabetes, high blood pressure or heart or circulation disease
- If you have an enlarged prostate
- If you have phaeochromocytoma or glaucoma
- If you are taking tricyclic antidepressants (e.g. imipramine or amitriptyline) or if you are taking or have taken within the last two weeks monoamine oxidase inhibitors (MAOIs) (e.g. moclobemide) prescribed for depression
- If you are taking beta blockers
- If your child is under 2 years of age
Take special care with Maxtra-P DS Syrup
- Contains paracetamol. Taking too much paracetamol can cause serious harm to your liver
- This product may cause dizziness. If affected do not drive or operate machinery.
- Do not drink alcohol (beer, wine, spirits etc) while taking this product.
Ask your doctor before you take this medicine
- If you have a blood vessel disease such as Raynaud’s Phenomenon
- If you have an enlarged prostate
- If you have epilepsy, heart or circulation disease, liver problems
- If you have a severe infection as this may increase the risk of metabolic acidosis
- Signs of metabolic acidosis include:
- Deep, rapid, difficult breathing
- Feeling sick (nausea), being sick (vomiting)
- Loss of appetite
- If you have high blood pressure or glaucoma
- If you have an overactive thyroid
- If you have difficulty passing urine
- If you have an obstruction in their intestine
- If you have a rare blood disease called porphyria
Contact a doctor immediately if you get a combination of these symptoms.
If you are taking other medicines
Talk to your doctor or pharmacist before taking Maxtra-P DS Syrup if you are taking any prescribed medicines; particularly
- Metoclopramide or domperidone (for nausea [feeling sick] or vomiting [being sick])
- Ergotamine or methylsergide (for migraine)
- Colestyramine (to lower blood cholesterol)
- Drugs to lower blood pressure
- Appetite suppressants or stimulants
- Drugs to treat depression such as tricyclic antidepressants (e.g. Amitriptyline) tranquillisers or other medicines for mental problems
- Drugs to treat heart disease (e.g. Digoxin)
- If you take blood thinning drugs (anticoagulants e.g. Warfarin)
- Other antihistamines
- Strong painkillers
- Sleeping tablets
- Phenytoin (for epilepsy)
- Atropine
Driving and using machines: This medicine may cause drowsiness. If affected do not drive or operate machinery. Avoid alcoholic drink
Pregnancy and breast feeding
Do not use if you are pregnant or breast feeding unless advised by a doctor.
3. How to take Maxtra-P DS Syrup
Check the seal is not broken before first use. If it is, do not give the medicine.
| 2 to 5 years of age | 2.5 ml every 4 to 6 hours |
|---|---|
| 6 to 11 years of age | 5 ml every 4 to 6 hours |
| ≥12 years of age | 10 ml every 4 to 6 hours |
Do not give to children under 2 years.
Do not give more than the amount recommended above.
If symptoms do not go away within 5 days talk to your pharmacist or doctor.
If you take too much: Talk to your doctor or go to your nearest hospital casualty department straight away. Take the medicine and this leaflet with you.
4. Possible side effects
Like all medicines, Maxtra-P DS Syrup can have side effects, but not everybody gets them. A small number of people have had side effects.
Stop taking this medicine and tell your doctor immediately if you experience:
- Allergic reactions which may be severe such as skin rash and itching sometimes with swelling of the mouth or face or shortness of breath.
- Very rare cases of serious skin reactions have been reported.
- Skin rash or peeling, or mouth ulcers, sensitivity to sunlight, other allergic reactions
- Breathing problems. These are more likely if you have experienced them before when taking other painkillers such as ibuprofen or aspirin.
- Unexplained bruising or bleeding.
- Reoccuring fevers or infections.
- Nausea, sudden weight loss, loss of appetite
- Visual disturbances. This is rare but is more likely in those with glaucoma.
- Difficulty passing water. This is more likely to occur in men with an enlarged prostate gland.
- Drowsiness (which may make your child fall asleep)
- Dizziness, blurred vision, headaches, fits
- Dry mouth, difficulty in passing urine, sweating
- Indigestion, stomach pain, loss of appetite
- Tremors, muscle pain or weakness, impaired movement or co-ordination, pins and needles
- Change in heart rate, palpitations, low blood pressure, ringing in the ears, hair loss
- Blood problems such as anaemia, weariness
- Sleep disturbance
- Liver problems (which may cause yellowing of the skin or eyes)
- Chest pain
- Cough, phlegm on the chest – these may be caused by thickened bronchial secretions (mucous) in your lungs
- Difficulty concentrating, irritability, depression
- Hyperactivity in children
These reactions are rare.
The following side effects may occur. Tell your doctor if you get them.
- Raised blood pressure, headache, dizziness, difficulty sleeping, nervousness, anxiety, diarrhoea or sickness.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Maxtra-P DS Syrup
Store at a temperature not exceeding 25oC
Keep out of reach of children.
Protect from light.
6. Contents of the pack and other information
What Maxtra-P DS Syrup contains
Each 5 ml contains:
Paracetamol 250 mg
Phenylephrine Hydrochloride 5 mg
Chlorpheniramine Maleate 2 mg
Packing
A bottle of 60 ml