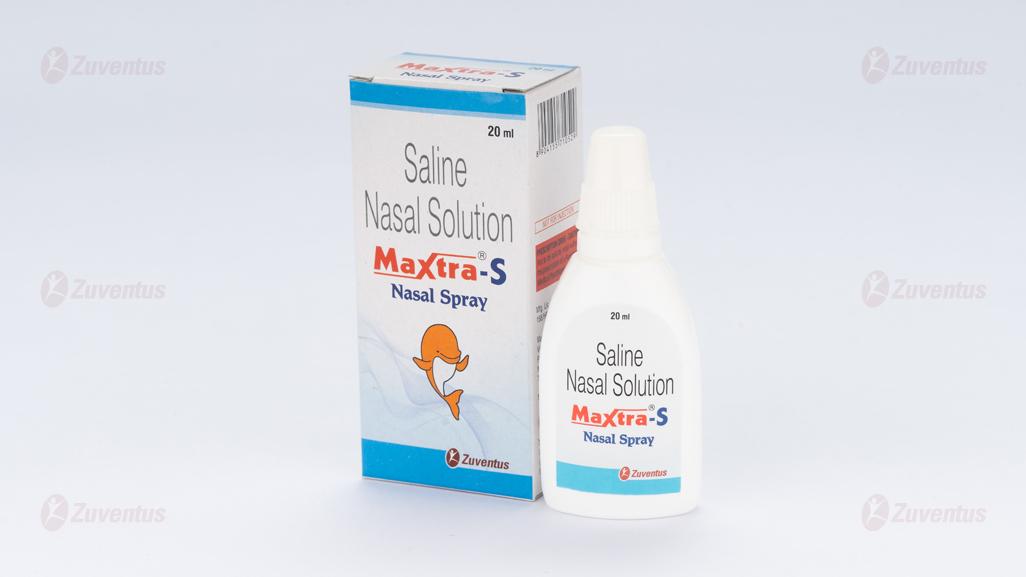
Maxtra S Nasal Spray
Therapy Area
Respiratory
1.0 Generic Name
Saline Nasal Solution IP
2.0 Qualitative and quantitative composition
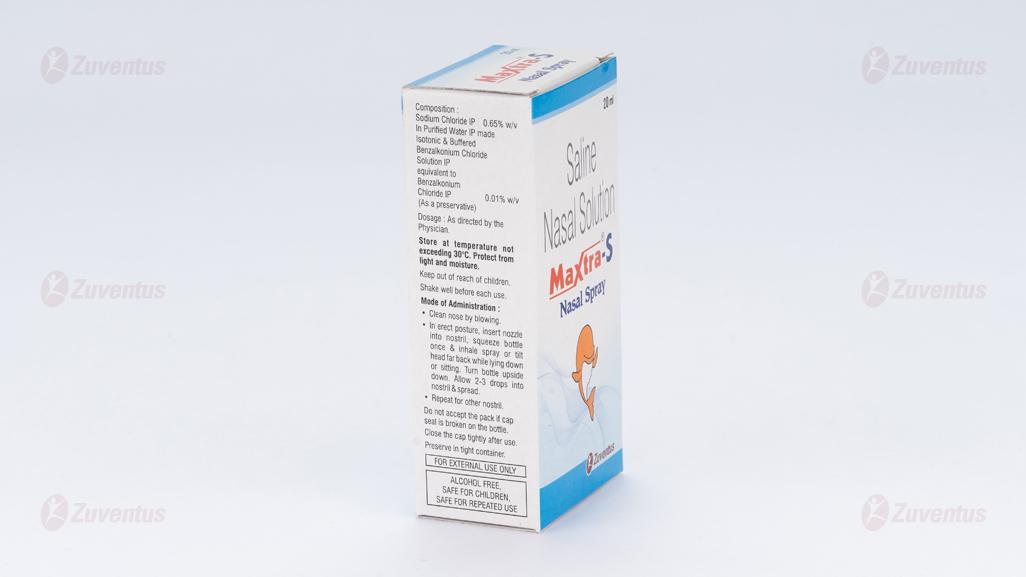
Sodium Chloride IP 0.65% w/v
In an Aqueous Isotonic Solution
using Purified Water IP
Excipients q.s.
Benzalkonium Chloride IP 0.01% w/v
(As a preservative)
3.0 Dosage form and strength
Nasal Spray
Sodium Chloride (0.65%w/v)
4.0 Clinical particulars
4.1 Therapeutic Indication
Naturally provides instant, soothing relief for dry, irritated nasal passages caused by colds, allergies, dry air, pollution, smoke, air travel, and the use of decongestants or steroidal sprays.
4.2 Posology and method of administration
Method of administration: Nasal use
- Clean nose by blowing.
- For children and adults: In erect posture, insert nozzle into nostril, squeeze bottle once & inhale spray.
- For infants/babies: tilt head far back while lying down or sitting. Turn bottle upside down. Allow 2-3 drops into nostril & spread.
- Repeat for other nostril
4.3 Contraindications
Maxtra-S Nasal Spray should not be used in the following conditions:
In case of hypersensitivity to the active substance or to any of the excipients listed in the formulation.
4.4 Special warnings and precautions for use
- The use of this Maxtra®-S Nasal Spray by more than one person may spread infection.
- Avoid if you are allergic to any of the ingredients in the formulation.
- Signs of an allergic reaction may include: rash, hives, itching, red-swollen-blistered or peeling skin with or without fever, wheezing, tightness in the chest or throat, trouble breathing, swallowing, unusual hoarseness or swelling of the mouth, face, lips, tongue or throat. Very severe nasal irritation.
- The product contains benzalkonium chloride which may cause irritation or swelling inside the nose, especially if used for a long time. Long term use may cause oedema of the nasal mucosa.
- Ensure that the container is undamaged and the contents clear in appearance before use. After use, discard any remaining solution.
4.5 Drugs interactions
None.
4.6 Use in special populations
Safe to use during pregnancy and lactation.
4.7 Effects on ability to drive and use machines
None known.
4.8 Undesirable effects
Usually do not occur, however rarely occurring side effects may include:
-if the inside of the nose is very dry and irritated, stinging may occur.
-allergic reactions
-sneezing
-cough
-nose irritation
-abnormal taste
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
None
5.0 Pharmacological properties
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Decongestants and other nasal preparations, sodium chloride nasal spray
ATC code: R01AX10
The exact mechanism of action of saline nasal irrigation is unknown. One possibility is that the breakdown of the protective function of the nasal mucosa plays a role in upper respiratory conditions. Saline nasal irrigation may improve nasal mucosa function through several physiologic effects, including direct cleansing, removal of inflammatory mediators and improved mucociliary function, as suggested by increasing ciliary beat frequency.
5.2 Pharmacokinetic properties
Absorption
Sodium chloride distributes primarily to extracellular compartments, including plasma and interstitial fluid; sodium is maintained outside the cell via the Na+/K+-ATPase pump, which exchanges intracellular sodium for extracellular potassium. Penetration across the blood-brain barrier is low. Sodium chloride is excreted primarily in the urine, but it is also excreted in sweat and stool. In healthy patients at steady state with minimal sweat losses, sodium excreted in urine is roughly the same as dietary intake. Sweat sodium concentration is increased in children with cystic fibrosis, aldosterone deficiency, or pseudohypoaldosteronism.
6.0 Nonclinical properties
No further information other than that which is included in the Summary of Product Characteristics.
6.1 Animal Toxicology or Pharmacology
No information provided.
7.0 Description
Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is listed on the World Health Organization Model List of Essential Medicine.
Molecular Weight : 58.44 g/mol.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None known.
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
A bottle of 20 ml.
8.4 Storage and handing instructions
Store at temperature not exceeding 30°C. Protect from light and moisture. Keep out of reach of children. Shake well before each use.
9.0 Patient Counselling Information
Do not accept the pack if cap seal is broken on the bottle.
Close the cap tightly after use.
Preserve in tight container.
Alcohol Free Safe for Children Safe for repeated daily use
12.0 Date of revision of text
18-09-2024
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
In this leaflet:
1. What Maxtra®-S Nasal Spray is and what it is used for
2. What you need to know before you take Maxtra®-S Nasal Spray
3. How to take Maxtra®-S Nasal Spray
4. Possible side effects
5. How to store Maxtra®-S Nasal Spray
6. Contents of the pack and other information
1. What Maxtra®-S Nasal Spray is and what it is used for
Maxtra®-S Nasal Spray contains Sodium Chloride (0.65%) known as normal saline which is a nasal decongestant & moisturizer for dry & stuffy nose.
Naturally it provides instant, soothing relief for dry, irritated nasal passages caused by colds, allergies, dry air, pollution, smoke, air travel, and the use of decongestants or steroidal sprays.
- It provides relief from nasal congestion and stuffy nose
- It helps moisturizes and loosen dried mucus in the nasal passages
- It can facilitate daily nasal hygiene
- This nasal spray provides relief from symptoms caused by allergies, sinus infections, and the common cold. Saline nasal irrigation may improve nasal mucosa function through several physiologic effects, including direct cleansing, removal of inflammatory mediators and improved mucociliary function, as suggested by increasing ciliary beat frequency.
2. Check before you take Maxtra®-S Nasal Spray
Do not take Maxtra®-S Nasal Spray:
If you are allergic to Sodium Chloride and Benzalkonium Chloride or any of the other ingredients of this medicine.
Talk to your doctor before you take this medicine
- The use of this Maxtra®-S Nasal Spray by more than one person may spread infection.
Driving and using machines: none known.
Pregnancy and breast feeding
Safe to use during pregnancy and lactation.
Important information about some of the ingredients in this medicine:
- Contains benzalkonium chloride which may cause irritation or swelling inside the nose, especially if used for a long time.
Tell your doctor if you have any known allergies.
3. How to take Maxtra-S Nasal Spray
For nasal use only

Method of administration: Nasal use
- Clean nose by blowing.
- For children and adults: In erect posture, insert nozzle into nostril, squeeze bottle once & inhale spray.
- For infants/babies: tilt head far back while lying down or sitting. Turn bottle upside down. Allow 2-3 drops into nostril & spread.
- Repeat for other nostril
4. Possible side effects
Usually do not occur, however rarely occurring side effects may include:
-if the inside of the nose is very dry and irritated, stinging may occur.
-allergic reactions
-sneezing
-cough
-nose irritation
-abnormal taste
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Maxtra-S Nasal Spray
Store at temperature not exceeding 30°C. Protect from light and moisture. Keep out of reach of children. Shake well before each use. Do not accept the pack if cap seal is broken on the bottle. Close the cap tightly after use. Preserve in tight container.
6. Contents of the pack and other information
What Maxtra-S Nasal Spray contains
Sodium Chloride IP 0.65% w/v
In an Aqueous Isotonic Solution
using Purified Water IP
Excipients q.s.
Benzalkonium
Chloride IP 0.01% w/v
(As a preservative)
Packing
A bottle of 20 ml.
Alcohol Free
Safe for Children
Safe for repeated daily use
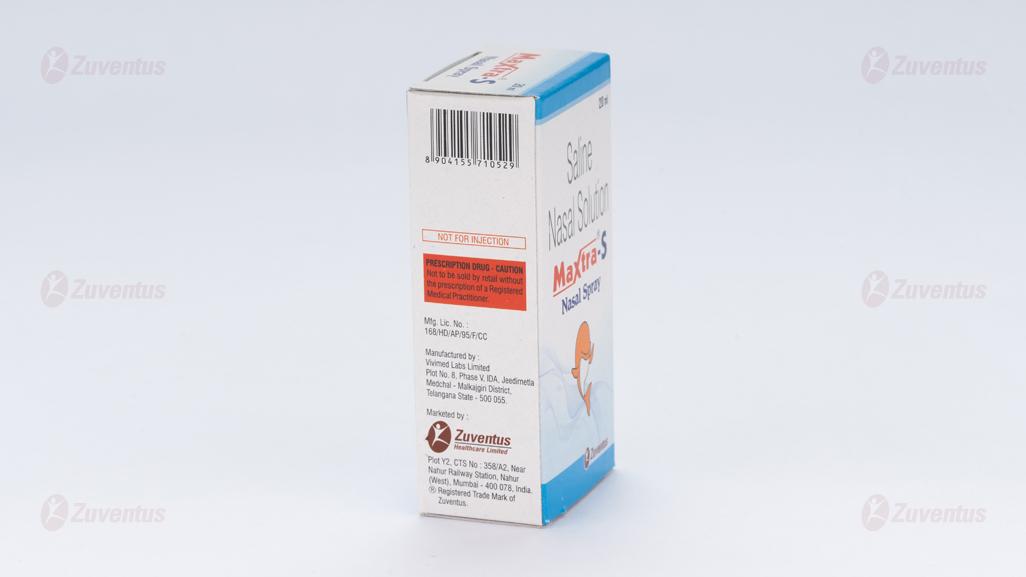
Marketing Authorisation Holder
Zuventus Healthcare Limited
Zuventus House, Plot Y2, CTS No.: 358/A2,
Near Nahur Railway Station,
Nahur (W), Mumbai, 400078 Maharashtra, India
This leaflet was last revised in 19/09/2024