Mefast 100 Suspension
Therapy Area
Pain management
1.0 Generic name
Mefenamic Acid Suspension
(Brand Name: Mefast 100 Suspension)
2.0 Qualitative and quantitative composition
Each 5 ml contains:
Mefenamic Acid IP 100 mg
Excipients q.s.
In a flavoured syrupy base
Colour: Sunset Yellow FCF
3.0 Dosage form and Strength
Dosage Form: Oral Suspension.
Dosage Strength: Mefenamic acid 100 mg per 5 ml suspension.
4.0 Clinical particulars
4.1 Therapeutic indication
- As an anti-inflammatory analgesic for the symptomatic relief of rheumatoid arthritis (including Still's Disease), osteoarthritis, and pain including muscular, traumatic and dental pain, headaches of most aetiology, post-operative and post-partum pain, pyrexia in children.
- Primary dysmenorrhoea in older children.
4.2 Posology and method of administration
Undesirable effects may be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms. Do not exceed the stated dose.
Paediatric population
It is recommended that children under 12 years of age should be given Mefenamic Acid Suspension (100mg/5ml) in the following dosage regime:
| Infants over 6 months | – 25mg/kg of bodyweight daily in 3 divided doses, or, |
| 6 months to under 2 years | - 2.5 ml |
| 2 years to under 5 years | – 5 ml |
| 5 years to under 9 years | - 7.5 ml |
| 9 years to 12 years | - 10 ml |
Doses may be repeated as necessary, up to three times daily.
Mefenamic acid suspension should be taken preferably with or after food.
Apart from the treatment of Still's Disease, therapy should not be continued for longer than 7 days in children.
Although mefenamic acid suspension is not indicated to be used by the elderly, if used in elderly patients, these patients are at increased risk of the serious consequences of adverse reactions. If an NSAID is considered necessary, the lowest effective dose should be used and for the shortest possible duration. The patient should be monitored regularly for GI bleeding during NSAID therapy.
Method of administration: For oral administration.
4.3 Contraindications
- Hypersensitivity to the active substance or any of the excipients.
- Inflammatory bowel disease
- History of gastrointestinal bleeding or perforation, related to previous NSAIDs therapy.
- Active, or history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding).
- Severe heart failure, hepatic failure and renal failure.
- Because the potential exists for cross-sensitivity to aspirin, ibuprofen, or other non-steroidal anti-inflammatory drugs, mefenamic acid must not be given to patients who have previously shown hypersensitivity reaction (e.g. asthma, bronchospasm, rhinitis, angioedema or urticaria) to these medicines.
- During the last trimester of pregnancy.
- Treatment of pain after coronary artery bypass graft (CABG) surgery.
4.4 Special warnings and precautions for use
Undesirable effects may be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms (see GI and cardiovascular risks below).
Patients on prolonged therapy should be kept under regular surveillance with particular attention to liver dysfunction, rash, blood dyscrasias or development of diarrhoea. Appearance of any of these symptoms should be regarded as an indication to stop therapy immediately
Use with concomitant NSAIDs including cyclooxygenase 2 specific inhibitors. Prolonged use of any type of painkiller for headaches can make them worse. If this situation is experienced or suspected, medical advice should be obtained and treatment should be discontinued. The diagnosis of 'Medication Overuse Headache' should be suspected in patients who have frequent or daily headaches despite (or because of) the regular use of headache medications.
Patients suffering from dehydration and renal disease particularly the elderly when mefenamic acid suspension has been considered appropriate in this age group.
Elderly
If used by the elderly, such patients have an increased frequency of adverse reactions to NSAIDs especially gastrointestinal bleeding and perforation which may be fatal.
Respiratory disorders
Caution is required if administered to patients suffering from, or with a previous history of, bronchial asthma since NSAIDs have been reported to precipitate bronchospasm in such patients.
Cardiovascular, renal and hepatic impairment
The administration of an NSAID may cause a dose dependant reduction in prostaglandin formation and precipitate renal failure. Patients at greatest risk of this reaction are those with impaired renal function, cardiac impairment, liver dysfunction, those taking diuretics and the elderly. Renal function should be monitored in these patients.
Cardiovascular and cerebrovascular effects
Appropriate monitoring and advice are required for patients with a history of hypertension and/or mild to moderate congestive heart failure as fluid retention and oedema have been reported in association with NSAID therapy.
Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long term treatment) may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke). There are insufficient data to exclude such a risk for mefenamic acid.
Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease should only be treated with mefenamic acid after careful consideration. Similar consideration should be made before initiating longer-term treatment of patients with risk factors for cardiovascular disease (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking).
As NSAIDs can interfere with platelet function, they should be used in caution in patients with intracranial haemorrhage and bleeding diathesis.
Gastrointestinal bleeding, ulceration and perforation
GI bleeding, ulceration or perforation, which can be fatal, has been reported with all NSAIDs at any time during treatment, with or without warning symptoms or a previous history of serious GI events. Smoking and alcohol use are added risk factors.
The risk of GI bleeding, ulceration or perforation is higher with increasing NSAID doses, in patients with a history of ulcer, particularly if complicated with haemorrhage or perforation, and in the elderly. Combination therapy with protective agents (e.g. misoprostol or proton pump inhibitors) should be considered for patients at risk of GI bleeding such as the elderly, and also for patients requiring concomitant low dose aspirin, or other drugs likely to increase gastrointestinal risk.
Patients with a history of GI toxicity, particularly when elderly, should report any unusual abdominal symptoms (especially GI bleeding) particularly in the initial stages of treatment.
Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors or anti-platelet agents such as aspirin.
When GI bleeding or ulceration occurs in patients receiving mefenamic acid the treatment should be withdrawn.
SLE and mixed connective tissue disease
In patients with systemic lupus erythematosus (SLE) and mixed connective tissue disorders there may be an increased risk of aseptic meningitis.
Skin reactions
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported in association with use of NSAIDs (see section 4.8). Patients appear to be at highest risk of these reactions early in the course of therapy, the onset of the reaction occurring in the majority of cases within the first month of treatment. Mefenamic acid should be stopped at the first appearance of skin rash, mucosal lesions or any other sign of hypersensitivity.
Female fertility
The use of mefenamic acid may impair female fertility and is not recommended in female patients who may be attempting to conceive. In female patients who may experience difficulties in conceiving or who are undergoing investigations of infertility withdrawal of mefenamic acid should be considered.
In dysmenorrhoea lack of response should alert the physician to investigate other causes.
Epilepsy
Caution should be exercised when treating patients suffering from epilepsy. In patients who are known or suspected to be poor CYP2C9 metabolisers based on previous history/experience with other CYP2C9 substrates, mefenamic acid should be administered with caution as they may have abnormally high plasma levels due to reduced metabolic clearance.
Alcohol
Concomitant consumption of alcohol with mefenamic acid may increase gastrointestinal bleeding, ulceration and perforation.
4.5 Drugs interactions
Concurrent therapy with other plasma protein binding drugs may necessitate a modification in dosage.
Anti-coagulants: NSAIDs may enhance the effects of anti-coagulants, such as warfarin. Concurrent administration of mefenamic acid with oral anti-coagulant drugs requires careful prothrombin time monitoring.
It is considered unsafe to take NSAIDs in combination with Warfarin or Heparin unless under direct medical supervision.
Lithium: a reduction in renal lithium clearance and elevation of plasma lithium levels. Patients should be observed carefully for signs of lithium toxicity.
The following interactions have been reported with NSAIDs but have not necessarily been associated with Mefenamic Acid Suspension:
Other analgesics including cyclooxygenase-2 selective inhibitors: avoid concomitant use of two or more NSAIDs (including aspirin) as this may increase the risk of adverse effects.
Antidepressants: selective serotonin reuptake inhibitors (SSRIs): increased risk of gastrointestinal bleeding.
Anti-hypertensives and diuretics: a reduction in antihypertensive and diuretic effect has been observed. Diuretics can increase the nephrotoxicity of NSAIDs.
ACE inhibitors and angiotensin-II-receptor antagonists: a reduction in antihypertensive effect and an increased risk of renal impairment especially in elderly patients. Patients should be adequately hydrated and the renal function assessed in the beginning and during concomitant therapy.
Aminoglycosides: reduction in renal function in susceptible individuals, decreased elimination of aminoglycoside and increased plasma concentrations.
Anti-platelet agents: increased risk of gastrointestinal ulceration or bleeding.
Acetylsalicylic Acid: experimental data implies that mefenamic acid interferes with the anti-platelet effect of low-dose aspirin when given concomitantly, and thus may interfere with aspirin's prophylactic treatment of cardiovascular disease. However, the limitations of this experimental data and the uncertainties regarding extrapolation of ex vivo data to the clinical situation imply that no firm conclusions can be made for regular mefenamic acid use.
Cardiac glycosides: NSAIDs may exacerbate cardiac failure, reduce GFR and increase plasma cardiac glycoside levels.
Ciclosporin: the risk of nephrotoxicity of ciclosporin may be increased with NSAIDs.
Corticosteroids: concomitant use may increase the risk of gastrointestinal ulceration or bleeding.
Oral hypoglycaemic agents: inhibition of metabolism of sulfonylurea drugs, prolonged half-life and increased risk of hypoglycaemia.
Methotrexate: elimination of the drug can be reduced, resulting in increased plasma levels.
Mifepristone: NSAIDs should not be taken for 8-12 days after mifepristone administration, NSAIDs can reduce the effects of mifepristone.
Probenecid: reduction in metabolism and elimination of NSAIDs and metabolites.
Quinolone antibiotics: animal data indicates that NSAIDs can increase the risk of convulsions associated with quinolone antibiotics. Patients taking NSAIDs and quinolones may have an increased risk of developing convulsions.
Tacrolimus: possible increased risk of nephrotoxicity when NSAIDS are given with tacrolimus.
Zidovudine: increased risk of haematological toxicity when NSAIDs are given with zidovudine. There is evidence of an increased risk of haemarthroses and haematoma in HIV(+) haemaophiliacs receiving concurrent treatment with zidovudine and ibuprofen.
4.6 Use in special populations
Pregnancy
Congenital abnormalities have been reported in association with NSAID administration in man; however, these are low in frequency and do not appear to follow any discernible pattern. In view of the known effects of NSAIDs on the foetal cardiovascular system (risk of closure of the ductus arteriosus), use in the last trimester of pregnancy is contraindicated. The onset of labour may be delayed and the duration increased with an increased bleeding tendency in both mother and child (See Contraindications). NSAIDs should not be used during the first two trimesters of pregnancy or labour unless the potential benefit to the patient outweighs the potential risk to the fetus. From the 20th week of pregnancy onward, mefenamic acid suspension use may cause oligohydramnios resulting from foetal renal dysfunction. This may occur shortly after treatment initiation and is usually reversible upon discontinuation. In addition, there have been reports of ductus arteriosus constriction following treatment in the second trimester, most of which resolved after treatment cessation.
If mefenamic acid is used by a woman attempting to conceive, or during the first and second trimester of pregnancy, the dose should be kept as low and duration of treatment as short as possible. Antenatal monitoring for oligohydramnios and ductus arteriosus constriction should be considered after exposure to mefenamic acid for several days from gestational week 20 onward. Mefenamic acid should be discontinued if oligohydramnios or ductus arteriosus constriction are found.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the foetus to:
- cardiopulmonary toxicity (with premature constriction/closure of the ductus arteriosus and pulmonary hypertension);
- renal dysfunction (see above); the mother and the neonate, at the end of pregnancy, to:
- possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses;
- inhibition of uterine contractions resulting in delayed or prolonged labour.
Labour and Delivery
inhibition of uterine contractions resulting in delayed or prolonged labour.
Fertility
See above section Special warnings and precautions for use regarding female fertility.
Nursing Mothers
Trace amounts of mefenamic acid may be present in breast milk and transmitted to the nursing infant. Therefore, mefenamic acid should not be taken by nursing mothers.
Geriatric use
The elderly patients have an increased frequency of adverse reactions to NSAIDs especially gastrointestinal (GI) bleeding and perforation which may be fatal. Thus, as like other NSAIDs, caution should be exercised while use of mefenamic acid in elderly population (> 65 years). Elderly patients with normal renal function may be given the same dose as recommended for adults. Mefenamic acid is known to be substantially excreted by the kidney, and the risk of toxic reactions to it may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
4.7 Effects on ability to drive and use machines
Undesirable effects such as dizziness, drowsiness, fatigue and visual disturbances are possible after taking NSAIDs. If affected, patients should not drive or operate machinery.
4.8 Undesirable effects
The most frequently reported side effects associated with mefenamic acid involve the gastrointestinal tract.
Diarrhoea occasionally occurs following the use of mefenamic acid. Although this may occur soon after starting treatment, it may also occur after several months of continuous use. The diarrhoea has been investigated in some patients who have continued this drug in spite of its continued presence. These patients were found to have associated proctocolitis. If diarrhoea does develop the drug should be withdrawn immediately and this patient should not receive mefenamic acid again.
Frequencies are not known for the following adverse reactions:
Blood and the lymphatic system disorders
Haemolytic anaemia*, anaemia, hypoplasia bone marrow, haematocrit decreased, thrombocytopenic purpura, temporary lowering of the white blood cell count (leukopenia) with a risk of infection, sepsis, and disseminated intravascular coagulation.
Agranulocytosis, aplastic anaemia, eosinophilia, neutropenia, pancytopenia, thrombocytopenia.
*reversible when mefenamic acid is stopped.
Immune system disorders
Hypersensitivity reactions have been reported following treatment with NSAIDs. These may consist of (a) non-specific allergic reactions and anaphylaxis (b) respiratory tract reactivity comprising asthma, aggravated asthma, bronchospasm, or dyspnoea or (c) assorted skin disorders including rashes of various types, pruritus, urticaria, purpura, angioedema, and more rarely exfoliative or bullous dermatoses (including epidermal necrolysis and erythema multiforme).
Metabolism and nutritional disorders
Glucose intolerance in diabetic patients, hyponatraemia.
Psychiatric disorders
Confusion, depression, hallucinations, nervousness.
Nervous system disorders
Optic neuritis, headaches, paraesthesia, dizziness, drowsiness, reports of aseptic meningitis (especially in patients with existing auto-immune disorders, such as systemic lupus erythematosus, mixed connective tissue disease), with symptoms such as stiff neck, headache, nausea, vomiting, fever or disorientation (see section 4.4).
Blurred vision, convulsions, insomnia.
Eye disorders
Eye irritation, reversible loss of colour vision, visual disturbances.
Ear and labyrinth disorders
Ear pain, tinnitus, vertigo.
Cardiac / Vascular disorders
Oedema, hypertension and cardiac failure have been reported in association with NSAID treatment. Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long term treatment) may be associated with an increased risk of arterial thrombotic events (for example myocardial infarction or stroke) (see section 4.4).
Palpitations.
Hypotension.
Respiratory, thoracic and mediastinal disorders
Asthma, dyspnoea.
Gastrointestinal disorders
The most commonly observed adverse events are gastrointestinal in nature. Peptic ulcers, perforation or GI bleeding, sometimes fatal, particularly in the elderly, may occur (see section 4.4). Nausea, vomiting, diarrhoea, flatulence, constipation, dyspepsia, abdominal pain, melaena, haematemesis, ulcerative stomatitis, exacerbation of colitis and Crohn's disease have been reported following administration. Less frequently, gastritis has been observed.
Elderly or debilitated patients seem to tolerate gastrointestinal ulceration or bleeding less well than other individuals and most spontaneous reports of fatal GI events are in this population. Anorexia, colitis, enterocolitis, gastric ulceration with or without haemorrhage, pancreatitis, steatorrhea.
Hepato-biliary disorders
Borderline elevations of one or more liver function tests, cholestatic jaundice. Mild hepatotoxicity, hepatitis, hepatorenal syndrome.
Skin and subcutaneous tissue disorders
Angioedema, laryngeal oedema, erythema multiforme, face oedema, bullous reactions including Lyell's syndrome (toxic epidermal necrolysis) and Stevens-Johnson syndrome, perspiration, rash, photosensitivity reaction, pruritus and urticaria.
Renal and urinary disorders
Allergic glomerulonephritis, acute interstitial nephritis, dysuria, haematuria, nephrotic syndrome, non- oliguric renal failure (particularly in dehydration), proteinuria, renal failure including renal papillary necrosis.
General disorders and administration site conditions
Fatigue, malaise, multi-organ failure, pyrexia.
Hypothermia has been reported in association with mefenamic acid, predominantly in paediatric patients.
Investigations
A positive reaction in certain tests for bile in the urine of patients receiving Mefenamic acid has been demonstrated to be due to the presence of the drug and its metabolites and not to the presence of bile.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine
4.9 Overdose
Symptoms
Symptoms include headache, nausea, vomiting, epigastric pain, gastrointestinal bleeding, rarely diarrhoea, disorientation, excitation, coma, drowsiness, tinnitus, fainting, occasionally convulsions [Mefenamic acid has a tendency to induce tonic-clonic (grand mal) convulsions in overdose]. In cases of significant poisoning acute renal failure and liver damage are possible.
Management
Patients should be treated symptomatically as required.
Within one hour of ingestion of a potentially toxic amount activated charcoal should be considered. Alternatively, in adults gastric lavage should be considered within one hour of ingestion of a potentially life-threatening overdose.
Good urine output should be ensured.
Renal and liver function should be closely monitored.
Patients should be observed for at least four hours after ingestion of potentially toxic amounts. Frequent or prolonged convulsions should be treated with intravenous diazepam.
Other measures may be indicated by the patient's clinical condition.
Haemodialysis is of little value since mefenamic acid and its metabolites are firmly bound to plasma proteins.
5.0 Pharmacological properties
5.1 Mechanism of action
Mefenamic acid has analgesic, anti-inflammatory, and antipyretic properties. The mechanism of action of mefenamic acid, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2). Mefenamic acid is a potent inhibitor of prostaglandin synthesis in vitro. Mefenamic acid concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because mefenamic acid is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
5.2 Pharmacodynamic Justification
Pharmacotherapeutic group: Anti-inflammatory and anti-rheumatic products, non-steroids, fenamates. ATC code: M01AG01.
Mefenamic acid is a non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic properties.
Its anti-inflammatory effect was first established in the UV erythema model of inflammation. Further studies included inhibition of granulation tissue growth into subcutaneous cotton pellets in rats and carrageenin induced rat paw oedema tests.
Antipyretic activity was demonstrated in yeast-induced pyresis in rats. In this model its antipyretic activity was roughly equal to that of phenylbutazone and flufenamic acid, but less than that of indomethacin. Analgesic activity was demonstrated in tests involving pain sensitivity of rats paws inflamed by brewers yeast. Mefenamic acid was less potent than flufenamic acid in this model.
Prostaglandins are implicated in a number of disease processes including inflammation, modulation of the pain response, dysmenorrhoea, menorrhagia and pyrexia.
In common with most NSAIDs mefenamic acid inhibits the action of prostaglandin synthetase (cyclo- oxygenase). This results in a reduction in the rate of prostaglandin synthesis and reduced prostaglandin levels.
The anti-inflammatory activity of NSAIDs in the rat paw oedema test has been correlated with their ability to inhibit prostaglandin synthetase. When mefenamic acid is ranked in both these tests it falls between indomethacin and phenylbutazone and it is probable that inhibition of prostaglandin synthesis contributes to the pharmacological activity and clinical efficacy of mefenamic acid.
There is also considerable evidence that the fenamates inhibit the action of prostaglandins after they have been formed. They therefore both inhibit the synthesis and response to prostaglandins. This double blockade may well be important in their mode of action.
Pharmacokinetic Justification
Absorption and distribution
Mefenamic acid is absorbed from the gastro intestinal tract. Peak levels of 10 mg/l occur two hours after the administration of a 1g oral dose to adults.
Biotransformation
Mefenamic acid is predominantly metabolised by cytochrome P450 enzyme CYP2C9 in the liver, first to a 3 hydroxymethyl derivative (metabolite I) and then a 3-carboxyl derivative (metabolite II). Both metabolites undergo secondary conjugation to form glucuronides.
Therefore, in patients who are known or suspected to be poor CYP2C9 metabolisers based on previous history/experience with other CYP2C9 substrates, mefenamic acid should be administered with caution as they may have abnormally high plasma levels due to reduced metabolic clearance.
Elimination
Fifty-two percent of a dose is recovered from the urine, 6% as mefenamic acid, 25% as metabolite I and 21% as metabolite II. Assay of stools over a 3-day period accounted for 10-20 % of the dose chiefly as unconjugated metabolite II.
The plasma levels of unconjugated mefenamic acid decline with a half-life of approximately two hours.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Preclinical safety data does not add anything of further significance to the prescriber.
7.0 Description
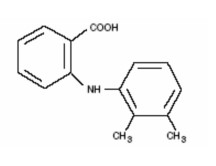
Mefenamic acid (2-(2,3-dimethylphenyl) aminobenzoic acid) is a non-steroidal anti-inflammatory drug (NSAIDs). Each 5 ml of Mefast 100 Suspension contains 100 mg of mefenamic acid for oral administration.
Mefenamic acid is a white to greyish-white, odorless, microcrystalline powder.
Molecular Weight: 241.29 g/mol.
Chemical Name: N-2,3-xylylanthranilic acid.
Molecular Formula: C15H15NO2.
Structural Formula:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf life
Refer on the pack.
8.3 Packaging information
60 ml bottle with measuring cup.
8.4 Storage and handling instructions
Store in a cool & dark place. Keep out of reach of children. SHAKE WELL BEFORE USE. SCHEDULE H PRESCRIPTION DRUG - CAUTION Not to be sold by retail without the prescription of a Registered Medical Practitioner.
9.0 Patient counselling information
Instructions to patients/caregivers:
- Ensure the prescribed dose of Mefast 100 Suspension is taken as directed. NSAID medicines should be used exactly as prescribed, at the lowest dose possible, and for the shortest time needed. Patients should not exceed the recommended dose or duration of treatment.
- Shake Mefast 100 suspension well before use each time.
- If pregnant or breast-feeding, ask a health professional before use. Instruct pregnant women not to take Mefast P Suspension especially in the last 3 months of pregnancy as it
- may cause harm to the foetus and during labour (delivery) because it may delay the labour and increases risk of bleeding.
- This medicine should not be given in children below 6 months of age.
- Do not administer this medicine if patient had an asthma attack, urticaria/itching, or other allergic reaction with aspirin or any other NSAID medicine.
- Administer this medicine after meal to reduce the stomach upset (heartburn).
- Ask the patient to report any adverse events.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
-Keep this leaflet. You may need to read it again.
-If you have any further questions, ask your doctor or pharmacist.
-This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
-If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1.What Mefast-100 Suspension is and what it is used for
2.What you need to know before you take Mefast-100 Suspension
3.How to take Mefast-100 Suspension
4.Possible side effects
5.How to store Mefast-100 Suspension
6.Contents of the pack and further information
1. What Mefast-100 Suspension is and what it is used for
Mefast-100 Suspension contains mefenamic acid which is a non-steroidal anti-inflammatory drug (NSAID).
This product is intended for children but may be given to adults.
It can help to relieve:
-symptoms of inflammation, such as redness and swelling
-pain and discomfort caused by arthritis, muscular or rheumatic disorders
-headache or toothache
-pain after operations, trauma
-fever in children painful or heavy periods in girls.
2.What you need to know before you take Mefast-100 Suspension
Do not take Mefast-100 Suspension
-if you are allergic to mefenamic acid, to any other anti-inflammatory medicines (such as aspirin, ibuprofen, celecoxib), or to any of the other ingredients (listed in section 6)
-if you have, or have ever had, stomach or intestinal conditions such as peptic ulcer, bleeding in the stomach or severe gastritis
-if you have an inflammatory bowel disease (e.g. ulcerative colitis, Crohn's disease)
-if you have severe heart, liver or kidney problems
- if you have just had heart bypass surgery
- if you are more than 6 months pregnant. If any of the above apply to you, talk to your doctor or pharmacist.
Warnings and precautions
Talk to your doctor or pharmacist before taking Mefast-100 Suspension:
-if you are taking any other NSAIDs (e.g. ibuprofen, diclofenac)
-if you are taking any other anti-inflammatory medicines including steroids (e.g. prednisolone)
-if you are taking aspirin or medicines that thin the blood (e.g. warfarin, clopidogrel)
-if you are taking antidepressants called selective serotonin re-uptake inhibitors (SSRIs) (e.g. paroxetine)
-if you have kidney or liver problems. Your doctor may check your kidney or liver function before and during treatment
-if you are elderly (see section 3)
-if you are trying to become pregnant (see section on Fertility)
-if you have stomach or digestive tract problems or if you ever had an upset stomach after taking pain killers such as aspirin. Bleeding in the stomach or gut can occur in patients taking Mefenamic Acid
-if you have a bleeding disorder or if you are going to have a major operation. Mefenamic Acid can affect the clotting of your blood. It can make you bleed more and for longer than usual
-if you have asthma, or a history of asthma, as this medicine may cause breathing difficulties
-if you have a connective tissue disorder, e.g. Systemic Lupus Erythematosus (SLE)
-if you have epilepsy
-if you are dehydrated (thirsty with dry skin, dark urine, dry mouth, headache)
-if you have heart problems, previous stroke or think that you might be at risk of these conditions (e.g. if you have high blood pressure, diabetes or high cholesterol or are a smoker). Additional monitoring may be carried out by your doctor.
Medicines such as Mefenamic Acid may be associated with a small increased risk of heart attack or stroke. Any risk is more likely with high doses and prolonged treatment. Do not exceed the recommended dose or duration of treatment.
Other medicines and Mefast-100 Suspension
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Some medicines may be affected by Mefenamic Acid or they may affect how well Mefenamic Acid will work. Tell your doctor or pharmacist if you are taking:
-medicines that can increase the chance of getting ulcers or a bleed in the stomach or gut, such as:
-corticosteroids used to treat arthritis and inflammation
-medicines such as anti-platelet agents, used to thin the blood (e.g. warfarin, aspirin, clopidogrel)
-antidepressants called selective serotonin re-uptake inhibitors (SSRIs) (e.g. paroxetine) -any other anti-inflammatory medicines (e.g. diclofenac, celecoxib)
-aspirin including low doses of aspirin used to prevent your blood from clotting in certain heart conditions
-medicines used for high blood pressure (e.g. atenolol, ramipril, valsartan)
-diuretics (water tablets) or heart medicines (e.g. digoxin, sotalol, diltiazem) -some diabetic medicines (e.g. glipizide, glibenclamide)
-medicines which suppress the immune system (e.g. ciclosporin, tacrolimus, methotrexate)
-lithium, a medicine used to treat mood swings and some types of depression
-a medicine usually prescribed through hospitals, called mifepristone (taken within the last 12 days)
-quinolone antibiotics (antibiotics used to treat infections)
-aminoglycoside antibiotics, used under medical supervision in hospitals
-zidovudine, a medicine used for HIV
-probenecid, a medicine used in special cases, to protect the kidneys
-medicines which bind to protein in the blood - (check with your pharmacist).
Blood tests
Your doctor may test your blood during treatment.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Pregnancy
Mefenamic acid will be passed to your unborn baby. You should not take Mefenamic Acid during the first 6 months of pregnancy unless absolutely necessary and advised by your doctor. If you need treatment during this period or while you are trying to get pregnant, the lowest dose for the shortest time possible should be used. If taken for more than a few days from 20 weeks of pregnancy onward, Mefenamic Acid can cause kidney problems in your unborn baby that may lead to low levels of amniotic fluid that surrounds the baby (oligohydramnios) or narrowing of a blood vessel (ductus arteriosus) in the heart of the baby. If you need treatment for longer than a few days, your doctor may recommend additional monitoring.
DO NOT take the Mefast-100 Suspension in the last 3 months of pregnancy as it may harm your unborn child and cause problems at delivery. It can cause kidney and heart problems in your unborn baby. It may affect your and your baby’s tendency to bleed and cause labour to be later or longer than expected.
Breast-feeding
Mefenamic acid passes into breast milk and can affect the baby. You should not take the suspension while breast-feeding unless advised by your doctor.
Fertility
DO NOT take the suspension if you are trying to become pregnant, as it may make it more difficult to get pregnant. You should inform your doctor if you are planning to become pregnant or if you have problems becoming pregnant.
Driving and using machines
Mefenamic acid may cause drowsiness, dizziness, fatigue or affect your vision. If any of these occur do not drive, use machinery, or perform any tasks that may require you to be alert.
3. How to take Mefast-100 Suspension
Always take this medicine exactly as your doctor has told you. Your doctor will decide on the appropriate dose to suit your condition. Check with your doctor or pharmacist if you are not sure.
-Shake the bottle before taking the medicine.
-Take the suspension with or immediately after a meal.
-After taking the suspension, rinse the mouth – this medicine contains sucrose which may be harmful to the teeth if taken for more than 2 weeks.
-Do not drink alcohol while taking Mefenamic Acid. Alcohol and smoking can irritate the stomach and make some of the side effects worse.
Dosages
Adults and the elderly
This medicine is intended for children. Contact your doctor for dosage advice if you are an adult taking this suspension.
Elderly patients are at a higher risk of side effects and should take the lowest effective dose for the shortest possible time, with additional monitoring carried out by their doctor.
Use in children
The recommended dose may be given up to 3 times per day.
| Age | Usual Dose |
| 6 months to 2 years | 2.5 ml |
| 2 years to 5 years | 5 ml |
| 5 years to 9 years | 7.5 ml |
| 9 years to 12 years | 10 ml |
Do not take Mefenamic Acid for more than 7 days unless advised to do so by your doctor.
If you take more Mefast-100 Suspension than you should
If you take more of the suspension than you should, you may harm your stomach, kidneys and you may get seizures (fits).
Symptoms of overdose may also include headache, nausea, stomach pain, vomiting, rarely diarrhoea, disorientation, excitation, coma (loss of consciousness for a period of time), tiredness, ringing in the ears, fainting and occasionally convulsions.
1.Tell your doctor, pharmacist or nearest hospital casualty department immediately.
2.Take the bottle and any remaining suspension with you so that people can see what you have taken.
3.Do this even if you feel well.
If you forget to take Mefast-100 Suspension
If you forget to take a dose, take it as soon as you remember, but if it is almost time for your next dose, skip the missed dose and continue as usual.
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Do not be alarmed by this list of possible side effects. You may not experience any of them.
Stop taking the suspension and seek medical help immediately if you have any of the following allergic reactions:
-difficulty breathing or swallowing, swelling of the face, lips, tongue or throat
-severe itching of the skin, with a red rash or raised lumps
-blistering of the mouth, eyes, and genital region, patchy areas of rash, peeling skin or any of the following reactions
-diarrhoea
-passing blood in your stools (faeces/motions)
-passing black tarry stools
-vomiting any blood or dark particles that look like coffee grounds.
Seek immediate medical attention if you have any of the following symptoms:
-indigestion or heartburn, abdominal pain (pain in your stomach) or other abnormal stomach symptoms, nausea (feeling sick), vomiting
-any unusual bruising or bleeding, for example nose-bleeds, pinpoint red spots on the skin, unusual purple bruise
-like rash on the skin or in the mouth
-signs of anaemia such as feeling tired, breathless, and looking pale
-fever, sore throat, mouth ulcers, repeated infections or infections that will not go away. This may be due to a low level of white blood cells
-seizures (fits)
-signs of low sodium levels such as headache, nausea, vomiting, tiredness, muscle cramps
-sudden headache, stiff neck, fever, sensitivity to bright light, drowsiness and muscle pain, with or without a rash
-fever, rash, nausea, aches and pains, passing more or less urine than usual, passing red urine or passing urine at night. This may be due to changes in your kidneys
-sudden loss or blurring of vision, loss of colour vision, eye pain which worsens with eye movement
-headache, in particular on waking in the morning. This may be due to high blood pressure
-pain behind the ribs radiating towards the back, often worse when lying down, nausea, vomiting, fever. This may be due to inflammation of your pancreas
-yellowing of your skin or eyes, pale faeces and dark urine, unexplained persistent nausea, stomach problems, loss of appetite or unusual tiredness. This may be due to changes in your liver
-low body temperature (below 35°C), feeling cold with pale-looking skin, especially in children.
The side effects listed below have been reported:
Not known: frequency cannot be estimated from the available data.
-head-spins (vertigo)
-hallucinations
-rapid heartbeat (palpitations)
-mental confusion
-constipation or bloating
-blurred vision, eye irritation
-feeling ill (malaise)
-ringing or buzzing in the ears (tinnitus)
-numbness or tingling in hands or feet
-sudden poor blood sugar control if you have diabetes. Your doctor or pharmacist can measure your sugar levels
-asthma or asthma that is worse than usual
-swelling of your hands and feet (around the ankles)
-sore mouth (pain or ulcers on the tongue, cheeks, lips, throat or gums)
-dizziness, drowsiness, feeling lethargic and tired
-signs of low blood pressure such as light-headedness
-reactions to the sun. Your skin may become red, painful and swollen
- do not sunbathe, use a sun bed, or expose your skin to artificial UV light.
-depression
-inability to sleep
-nervousness
-sweating
-fatty stools
- loss of appetite
- ear pain.
Medicines such as Mefenamic Acid may be associated with a small increased risk of heart attack or stroke. (see section 2 - end of 'Warnings and precautions').
Urine tests
Tell the doctor if you are having urine tests, as your medicine may affect the results.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Mefast-100 Suspension
Keep this medicine out of the sight and reach of children. Do not take after the expiry date which is stated on the bottle label and on the carton after EXP. The expiry date refers to the last day of that month. Store below 30°C. Do not throw away any medicine via wastewater or household waste. Ask your pharmacist how to throw away medicine you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Mefast-100 Suspension contains
The active substance is mefenamic acid (100 mg per 5 ml).
Each 5 ml contains:
Acid IP 100 mg
Excipients q.s.
In a flavoured syrupy base
Colour: Sunset Yellow FCF
What Mefast-100 Suspension looks like and contents of the pack
Mefenamic Acid Suspension is an off-white suspension with typical aroma and taste.
60 ml bottle with measuring cup.