Merotec XP 1.5g Injection
Therapy Area
Anti Infective
1.0 Generic name
Meropenem and Sulbactam for Injection
2.0 Meropenem and Sulbactam for Injection
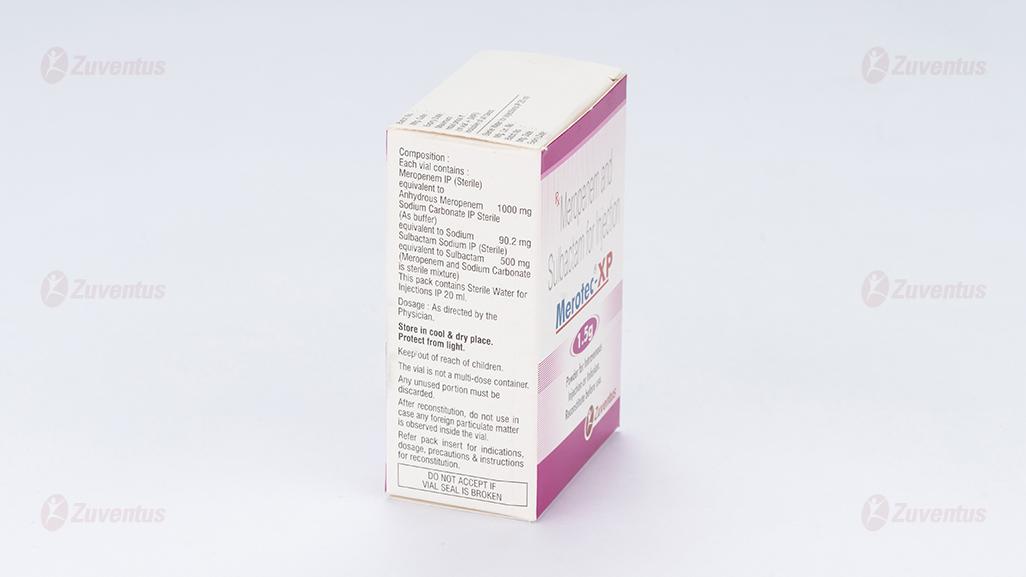
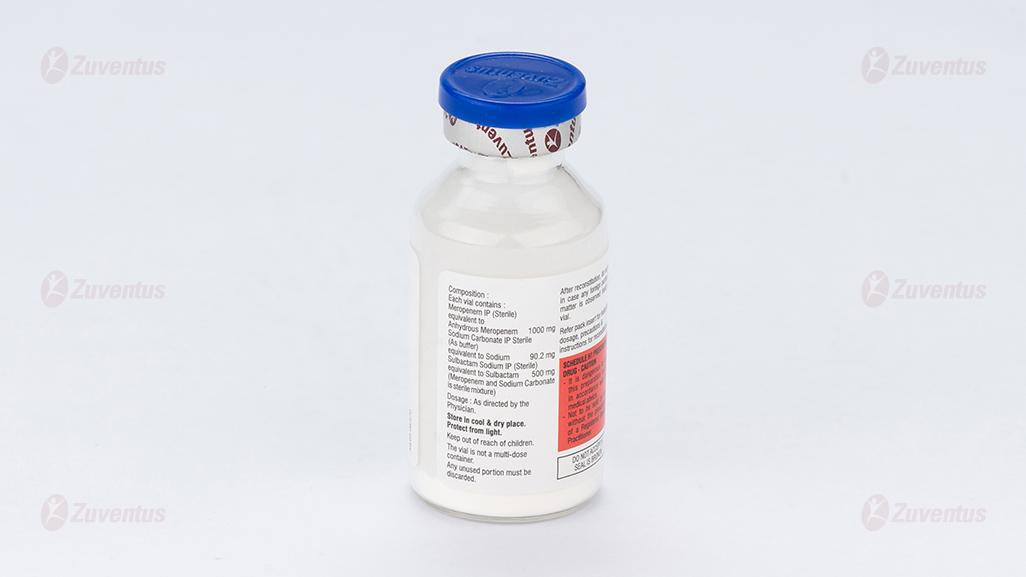
Each vial contains:
Meropenem IP (Sterile) equivalent to Anhydrous Meropenem 1000 mg
Sodium Carbonate IP Sterile (As buffer) equivalent to Sodium 90.2 mg
Sulbactam Sodium IP (Sterile)equivalent to Sulbactam 500 mg
(Meropenem and Sodium Carbonate is sterile mixture)
This pack contains Sterile Water for Injections IP 20 ml.
3.0 Dosage form and strength
Powder for intravenous injection or infusion
1.5 g/vial
4.0 Clinical Particulars
4.1 Therapeutic indication
Merotec-XP is indicated for treatment of infections caused by single or multiple bacteria originally sensitive to Meropenem.
- Pneumonia, including community acquired pneumonia and nosocomial pneumonia.
- Urinary Tract Infections.
- Intra-abdominal Infections.
- Gynaecological Infections such as endometritis and pelvic inflammatory disease.
- Skin and Skin Structure Infections.
- Meningitis.
- Septicemia.
- Empiric treatment, for presumed infections in adult patients with febrile neutropenia, used as monotherapy or in combination with anti-viral or anti-fungal agents. Meropenem has proved to be efficacious alone or in combination with other antimicrobial agents in the treatment of polymicrobial infections.
4.2 Posology and method of administration
Adults
The dosage and duration of therapy depends on the type and severity of infection and the condition of the patient.
Dosages are expressed in terms of Meropenem:
- 500 mg IV every 8 hours in the treatment of pneumonia, UTI, gynaecological infections such as endometritis, skin and skin structure infections.
- 1 gm IV every 8 hours in the treatment of nosocomial pneumonia, peritonitis, presumed infections in neutropenic patients, septicaemia.
- In meningitis the recommended dosage is 2 gm every 8 hours.
As with other antibiotics, caution is recommended in using Meropenem as monotherapy in critically ill patients with known or suspected Pseudomonas aeruginosa lower respiratory tract infection.
Regular sensitivity testing is recommended when treating Pseudomonas aeruginosa infection.
Dosage Schedule for Adults with impaired Renal Function
Dosage should be reduced in patients with creatinine clearance less than 51 ml/min. as planned below.
| Creatinine Clearance (ml/min) | Dose | Frequency |
| 26-50 | One unit dose | every 12 hours |
| 10-25 | One-half unit dose | every 12 hours |
| <10 | One-half unit dose | every 24 hours |
Meropenem is cleared by hemodialysis; if continued treatment with Meropenem is necessary, it is recommended that the unit dose (based on the type and severity of infection) is administered at the completion of the hemodialysis procedure to restore therapeutically effective plasma concentrations.
There is no experience with the use of Meropenem in patients under peritoneal dialysis.
Adults with Hepatic Insufficiency
Elderly Patients
No dosage adjustment is required for elderly with normal renal function or creatinine clearance values above 50 ml/min.
Children
For children ≥ 3 months and up to 12 years of age the recommended dose is 10-20 mg/kg every 8 hours depending on type and severity of infection, susceptibility of the pathogen and the condition of the patient. In children over 50 kg weight, adult dosage should be used. In meningitis the recommended dose is 40 mg/kg ever y 8 hours. Safety and efficacy of Meropenem in children with renal impairment has not been established.
Method of Administration
Merotec-XP for Injection can be given as an I.V. bolus injection over approximately 5 minutes, or by I.V. infusion over approximately 15 to 30 minutes, using the specific available diluents.
Preparations of Solutions:
For Intravenous Bolus Administration:
Constitute injection vial (1 gm Meropenem and 500 mg Sulbactam) with Sterile water for Injections IP (See table below). Shake to dissolve and let it stand until clear.
| Vial size | Amount of Diluent Added (ml) | Approximate withdrawable volume (ml) | Approximate Average Concentration (mg/ml of Meropenem) |
| 1.5 gm | 20 | 20 | 50 |
For Infusion:
Infusion vials (1.5 gm) may be directly constituted with a compatible infusion fluid. Alternatively, an injection vial may be constituted, then the resulting solution to an I.V. container and further diluted with an appropriate infusion fluid.
4.3 Contraindications
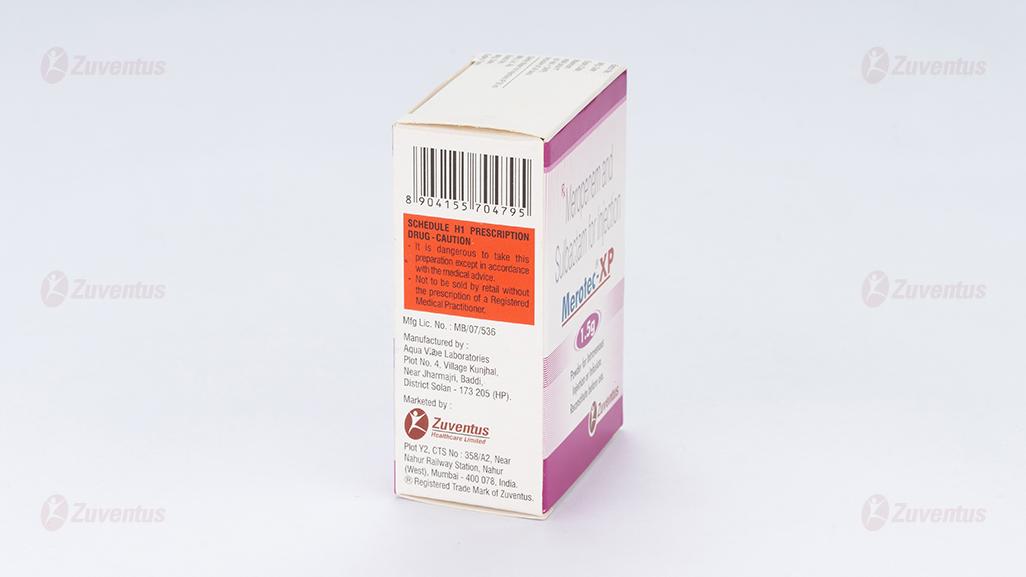
Merotec-XP is contraindicated in patients:
- Hypersensitivity to the active substance or to any of the excipients.
- Hypersensitivity to any other carbapenem antibacterial agent.
- Severe hypersensitivity (e.g. anaphylactic reaction, severe skin reaction) to any other type of betalactam antibacterial agent (e.g. penicillins or cephalosporins).
4.4 Special warnings and precautions for use
Hypersensitivity Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving therapy with β-lactams. Before initiating therapy with Merotec-XP, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, other β-lactams, and other allergens. Seizure Potential: Seizures and other adverse CNS experiences have been reported during treatment with Merotec-XP. These experiences have occurred most commonly in patients with CNS disorders (e.g., brain lesions/seizures) or with bacterial meningitis and/or compromised renal function. If focal tremors, myoclonus, or seizures occur, patients should be evaluated neurologically, placed on anticonvulsant therapy and the dosage of Merotec-XP re-examined to determine whether it should be decreased or the antibiotic discontinued.
Interaction with Valproic Acid: Co-administration of carbapenems, including Meropenem, to patients receiving valproic acid results in a reduction in valproic acid concentrations and thereby increasing the risk of breakthrough seizures. The concomitant use of Merotec-XP and valproic acid or divalproex sodium is generally not recommended.
Clostridium difficile-Associated Diarrhea: Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Merotec-XP, and may range in severity from mild diarrhea to fatal colitis. If CDAD is suspected or confirmed, ongoing antibiotic use may need to be discontinued.
Overgrowth of Nonsusceptible Organisms: As with other broad-spectrum antibiotics, prolonged use of Merotec-XP may result in overgrowth of nonsusceptible organisms. Repeated evaluation of the patient is essential. If super infection does occur during therapy, appropriate measures should be taken.
Laboratory Tests: While Merotec-XP possesses the characteristic low toxicity of the beta-lactam group of antibiotics, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during prolonged therapy.
Patients with Renal Impairment: In patients with renal impairment, thrombocytopenia has been observed but no clinical bleeding reported.
4.5 Drugs interactions
Probenecid competes with Meropenem for active tubular secretion and thus inhibits the renal excretion of Meropenem. Following administration of probenecid with Meropenem, the mean systemic exposure increased 56% and the mean elimination half-life increased 38%. Co-administration of probenecid with Meropenem is not recommended.
- Probenecid competes with Meropenem for active tubular secretion and thus inhibits the renal excretion, with the effect of increasing the elimination half-life and plasma concentration of Meropenem. Co-administration of probenecid with Merotec-XP is not recommended.
- Merotec-XP may reduce serum Valproic acid levels. Sub therapeutic levels may be reached in some patients.
4.6 Use in special populations
Pregnancy:
Pregnancy Category B There are no adequate and well-controlled studies in pregnant women. Merotec-XP should be used during pregnancy only if clearly needed.
Paediatric Use:
The safety and effectiveness of Meropenem has been established for paediatric patients ≥ 3 months of age. Use of Meropenem for injection in paediatric patients with bacterial meningitis, intra-abdominal infections, complicated skin and skin structure infections is supported by evidence from an adequate and well-controlled study in adults and additional data from paediatric pharmacokinetics studies.
Lactation:
Meropenem is detectable at very low concentrations in animal breast milk. Merotec-XP should not be used in breast-feeding women unless the benefit justifies the potential risk to the baby.
Geriatric Use:
Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Hepatic Insufficiency:
No dosage adjustment is necessary in adult patients with impaired hepatic function. There is no experience in pediatric patients with hepatic impairment.
4.7 Effects on ability to drive and use machines
No studies on the effect on the ability to drive and use machines have been performed. However, when driving or operating machines, it should be taken into account that headache, paraesthesia and convulsions have been reported for Meropenem.
4.8 Undesirable effects
Summary of the safety profile
In a review of 4,872 patients with 5,026 meropenem treatment exposures, meropenem-related adverse reactions most frequently reported were diarrhoea (2.3 %), rash (1.4 %), nausea/vomiting (1.4 %) and injection site inflammation (1.1 %).
The most commonly reported meropenem-related laboratory adverse events were thrombocytosis (1.6 %) and increased hepatic enzymes (1.5-4.3 %).
Tabulated risk of adverse reactions
In the table below all adverse reactions are listed by system organ class and frequency: very common (≥ 1/10); common (≥ 1/100 to <1/10); uncommon (≥ 1/1,000 to <1/100); rare (≥ 1/10,000 to <1/1,000); very rare (< 1/10,000); not known (cannot be estimated from the available data. Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.

Description of selected adverse reactions
Kounis syndrome
Acute coronary syndrome associated with an allergic reaction (Kounis syndrome) has been reported with other beta-lactam antibiotics.
Paediatric population
Meropenem is licensed for children over 3 months of age. There is no evidence of an increased risk of any adverse drug reaction in children based on the limited available data. All reports received were consistent with events observed in the adult population.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Relative overdose may be possible in patients with renal impairment if the dose is not adjusted. Limited post-marketing experience indicates that if adverse reactions occur following overdose, they are consistent with the adverse reaction profile described as above, are generally mild in severity and resolve on withdrawal or dose reduction. Symptomatic treatments should be considered.
In individuals with normal renal function, rapid renal elimination will occur.
Haemodialysis will remove meropenem and its metabolite.
5.0 Pharmacological Properties
Pharmacotherapeutic group: antibacterials for systemic use, carbapenems
ATC code: J01DH02
5.1 Mechanism of Action
Meropenem
Meropenem is a broad-spectrum carbapenem antibiotic. The bactericidal activity of Meropenem results from the inhibition of cell wall synthesis. Meropenem readily penetrates the cell wall of most Gram-positive and Gram-negative bacteria to reach penicillin-binding-protein (PBP) targets. Its strongest affinities are towards PBP 2, 3 and 4 of Escherichia coli and Pseudomonas aeruginosa; and PBP 1, 2 and 4 of Staphylococcus aureus. Bactericidal concentrations are typically 1-2 times the bacteriostatic concentrations of Meropenem, with the exception of Listeria monocytogenes, against which lethal activity is not observed. Meropenem should not be used to treat methicillin-resistant Staphylococcus aureus (MRSA) or methicillin-resistant Staphylococcus epidermidis (MRSE).
Sulbactam
Sulbactam is a β-lactamase inhibitor. It is active against Neisseriaceae and Acinetobacter baumanii, but generally has only weak antibacterial activity against other organisms. It is an irreversible inhibitor of many plasmid-mediated and chromosomal beta lactamases found in microorganisms resistant to penicillins and cephalosporins. Sulbactam has greater activity (MIC90 = 7.71 mg/ml) against A.baumannii (a common isolate from mixed infections, mainly involved in hospital-acquired infections like ventilator acquired pneumonia and in community-acquired infections) than either tazobactam (MIC90 = 22.10 mg/ml) or clavulanic acid (MIC90 = 28.40 mg/ml).
5.2 Pharmacodynamic properties
Meropenem is a β-lactam antibiotic, presently considered as the most potent agent for treatment of multidrug resistant gram-negative infections. Recently there have been reports of resistance to carbapenems. Emergence of Meropenem resistance has been reported due to development of mutant plasmid mediated metallo-β-lactamases (IMP-6), AmpC β–lactamases & certain ESBLs i.e. CTX-M. This is of great concern as carbapenems are considered the last resort antibiotics presently to combat infections by multidrug resistant bacteria especially in intensive care units.
Resistance significantly limits treatment options for life-threatening infections caused by gram negative bacteria.
Sulbactam, a β-lactamase inhibitor, increases antimicrobial activity of Meropenem by inhibiting the enzyme β-lactamase. Sulbactam has been reported to extend spectrum of activity of several antibiotics & has higher stability in the solution compared to clavulanate. Fixed dose Combination of Meropenem and Sulbactam in the ratio of 2:1 exhibited the synergistic activity against multidrug resistant Acinetobacter baumannii, E. coli and Pseudomonas aeruginosa isolates.
Therefore, Merotec-XP is a justified fixed dose combination to increase the spectrum of activity of Meropenem and prevent the emergence of resistant strains.
Pharmacokinetic/Pharmacodynamic (PK/PD) relationship
Similar to other beta-lactam antibacterial agents, the time that meropenem concentrations exceed the MIC (T>MIC) has been shown to best correlate with efficacy. In preclinical models meropenem demonstrated activity when plasma concentrations exceeded the MIC of the infecting organisms for approximately 40 % of the dosing interval. This target has not been established clinically.
Mechanism of resistance
Bacterial resistance to meropenem may result from: (1) decreased permeability of the outer membrane of Gram-negative bacteria (due to diminished production of porins) (2) reduced affinity of the target PBPs (3) increased expression of efflux pump components, and (4) production of beta-lactamases that can hydrolyse carbapenems.
Localised clusters of infections due to carbapenem-resistant bacteria have been reported in the European Union.
There is no target-based cross-resistance between meropenem and agents of the quinolone, aminoglycoside, macrolide and tetracycline classes. However, bacteria may exhibit resistance to more than one class of antibacterials agents when the mechanism involved include impermeability and/or an efflux pump(s).
Breakpoints
MIC (minimum inhibitory concentraztion) interpretive criteria for susceptibility testing have been established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for meropenem and are listed below:
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
The following table of pathogens listed is derived from clinical experience and therapeutic guidelines.
Commonly susceptible species
Gram-positive aerobes
Enterococcus faecalis$
Staphylococcus aureus (methicillin-susceptible)£
Staphylococcus species (methicillin-susceptible) including
Staphylococcus epidermidis
Streptococcus agalactiae (Group B)
Streptococcus milleri group (S. anginosus, S. constellatus, and S. intermedius)
Streptococcus pneumoniae
Streptococcus pyogenes (Group A)
Gram-negative aerobes
Citrobacter freundii
Citrobacter koseri
Enterobacter aerogenes
Enterobacter cloacae
Escherichia coli
Haemophilus influenzae
Klebsiella oxytoca
Klebsiella pneumoniae
Morganella morganii
Neisseria meningitidis
Proteus mirabilis
Proteus vulgaris
Serratia marcescens
Gram-positive anaerobes
Clostridium perfringens
Peptoniphilus asaccharolyticus
Peptostreptococcus species
(including P. micros, P anaerobius, P. magnus)
Gram-negative anaerobes
Bacteroides caccae
Bacteroides fragilis group
Prevotella bivia Prevotella disiens
Species for which acquired resistance may be a problem
Gram-positive aerobes
Enterococcus faecium$†
Gram-negative aerobes
Acinetobacter species
Burkholderia cepacia
Pseudomonas aeruginosa
Inherently resistant organisms
Gram-negative aerobes
Stenotrophomonas maltophilia
Legionella species
Other micro-organisms
Chlamydophila pneumoniae
Chlamydophila psittaci
Coxiella burnetii
Mycoplasma pneumoniae
$ Species that show natural intermediate susceptibility
£ All methicillin-resistant staphylococci are resistant to meropenem
† Resistance rate ≥ 50% in one or more EU countries.
Glanders and melioidosis: Use of meropenem in humans is based on in vitro B.mallei and B. pseudomallei susceptibility data and on limited human data. Treating physicians should refer to national and/or international consensus documents regarding the treatment of glanders and melioidosis.
5.3 Pharmacokinetic properties
Absorption
In healthy subjects the mean plasma half-life is approximately 1 hour; the mean volume of distribution is approximately 0.25 l/kg (11-27 l) and the mean clearance is 287 ml/min at 250 mg falling to 205 ml/min at 2 g.
Doses of 500, 1000 and 2000 mg doses infused over 30 minutes give mean Cmax values of approximately 23, 49 and 115 μ g/ml respectively, corresponding AUC values were 39.3, 62.3 and 153 μ g.h/ml. After infusion over 5 minutes Cmax values are 52 and 112 μ g/ml after 500 and 1000 mg doses respectively. When multiple doses are administered 8-hourly to subjects with normal renal function, accumulation of meropenem does not occur.
A study of 12 patients administered meropenem 1000 mg 8 hourly post-surgically for intra-abdominal infections showed a comparable Cmax and half-life to normal subjects but a greater volume of distribution 27 l.
Distribution
The average plasma protein binding of meropenem was approximately 2 % and was independent of concentration. After rapid administration (5 minutes or less) the pharmacokinetics are biexponential but this is much less evident after 30 minutes infusion. Meropenem has been shown to penetrate well into several body fluids and tissues: including lung, bronchial secretions, bile, cerebrospinal fluid, gynaecological tissues, skin, fascia, muscle, and peritoneal exudates.
Biotransformation
Meropenem is metabolised by hydrolysis of the beta-lactam ring generating a microbiologically inactive metabolite. In vitro meropenem shows reduced susceptibility to hydrolysis by human dehydropeptidase-I (DHP-I) compared to imipenem and there is no requirement to co-administer a DHP-I inhibitor.
Elimination
Meropenem is primarily excreted unchanged by the kidneys; approximately 70 % (50 – 75 %) of the dose is excreted unchanged within 12 hours. A further 28% is recovered as the microbiologically inactive metabolite. Faecal elimination represents only approximately 2% of the dose. The measured renal clearance and the effect of probenecid show that meropenem undergoes both filtration and tubular secretion.
Renal insufficiency
Renal impairment results in higher plasma AUC and longer half-life for meropenem. There were AUC increases of 2.4 fold in patients with moderate impairment (CrCL 33-74 ml/min), 5 fold in severe impairment (CrCL 4-23 ml/min) and 10 fold in haemodialysis patients (CrCL <2 ml/min) when compared to healthy subjects (CrCL >80 ml/min). The AUC of the microbiologically inactive ring opened metabolite was also considerably increased in patients with renal impairment. Dose adjustment is recommended for patients with moderate and severe renal impairment (see section 4.2).
Meropenem is cleared by haemodialysis with clearance during haemodialysis being approximately 4 times higher than in anuric patients.
Hepatic insufficiency
A study in patients with alcoholic cirrhosis shows no effect of liver disease on the pharmacokinetics of meropenem after repeated doses.
Adult patients
Pharmacokinetic studies performed in patients have not shown significant pharmacokinetic differences versus healthy subjects with equivalent renal function. A population model developed from data in 79 patients with intra-abdominal infection or pneumonia, showed a dependence of the central volume on weight and the clearance on creatinine clearance and age.
Paediatric population
The pharmacokinetics in infants and children with infection at doses of 10, 20 and 40 mg/kg showed Cmax values approximating to those in adults following 500, 1000 and 2000 mg doses, respectively. Comparison showed consistent pharmacokinetics between the doses and half-lives similar to those observed in adults in all but the youngest subjects (<6 months t1/2 1.6 hours). The mean meropenem clearance values were 5.8 ml/min/kg (6-12 years), 6.2 ml/min/kg (2-5 years), 5.3 ml/min/kg (6-23 months) and 4.3 ml/min/kg (2-5 months). Approximately 60 % of the dose is excreted in urine over 12 hours as meropenem with a further 12 % as metabolite. Meropenem concentrations in the CSF of children with meningitis are approximately 20 % of concurrent plasma levels although there is significant inter-individual variability.
The pharmacokinetics of meropenem in neonates requiring anti-infective treatment showed greater clearance in neonates with higher chronological or gestational age with an overall average half-life of 2.9 hours. Monte Carlo simulation based on a population PK model showed that a dose regimen of 20 mg/kg 8 hourly achieved 60 %T>MIC for P. aeruginosa in 95 % of pre-term and 91 % of full term neonates.
Elderly
Pharmacokinetic studies in healthy elderly subjects (65-80 years) have shown a reduction in plasma clearance, which correlated with age-associated reduction in creatinine clearance, and a smaller reduction in non-renal clearance. No dose adjustment is required in elderly patients, except in cases of moderate to severe renal impairment.
Sulbactam
Sulbactam has pharmacokinetic characteristics in humans similar to those of ampicillin and amoxicillin. After a 30-min infusion of 500 mg of sulbactam, a peak serum concentration of approximately 20μg/ml was obtained; 1000 mg produced a peak of 43 μg/ml. The half-life for elimination was about 1.0 hour. The data were interpreted as a two-compartment pharmacokinetic model. The results (table given below) are compatible with i.v. infusion followed by moderate rates of distribution into and out of a peripheral compartment (k12 = 0.9 h-1; k21= 1.8 h) and a moderate elimination rate (k10 = 1.4 h). The apparent volume of distribution for the central compartment (serum and rapidly equilibrating tissues) was between 9 and 16 liters, and the total apparent volume of distribution was between 19 and 28 liters. Approximately 51% of the drug was in the central compartment in the post distributive phase.
Calculated steady-state serum concentrations were proportional to dose. The results show that parenteral Sulbactam acts like a typical penicillin in humans. Sulbactam when administered to humans by bolus i.v. injections: 500 mg produced a peak serum concentration of approximately 32 μg/ml.
In this study, approximately 70% of a parenteral dose of sulbactam was excreted in the urine between 0 and 6 hours, with an additional recovery of 5 % between 6 and 12 hours post dose.
Pharmacokinetic parameters of sulbactam in humans: two compartment open model, 30 min i.v. infusion.

6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Animal studies indicate that meropenem is well tolerated by the kidney. Histological evidence of renal tubular damage was seen in mice and dogs only at doses of 2000 mg/kg and above after a single administration and above and in monkeys at 500 mg/kg in a 7-day study.
Meropenem is generally well tolerated by the central nervous system. Effects were seen in acute toxicity studies in rodent at doses exceeding 1000 mg/kg.
The IV LD50 of meropenem in rodents is greater than 2000 mg/kg.
In repeat dose studies of up to 6 months duration only minor effects were seen including a decrease in red cell parameters in dogs.
There was no evidence of mutagenic potential in a conventional test battery and no evidence of reproductive toxicity including teratogenic potential in studies in rats up to 750 mg/kg and in monkeys up to 360 mg/kg.
There was no evidence of increased sensitivity to meropenem in juveniles compared to adult animals. The intravenous formulation was well tolerated in animal studies.
The sole metabolite of meropenem had a similar profile of toxicity in animal studies.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Merotec XP is and what it is used for
2. What you need to know before you take Merotec XP
3. How to take Merotec XP
4. Possible side effects
5. How to store Merotec XP
6. Contents of the pack and other information
1. What Merotec XP is and what it is used for
Merotec XP is the fixed-dose combination of Meropenem and sulbactam in a ratio of 2:1. Meropenem belongs to a group of medicines called carbapenem antibiotics. It works by killing bacteria, which can cause serious infections. Sulbactam, a β-lactamase inhibitor, increases the antimicrobial activity of Meropenem by inhibiting the enzyme β-lactamase.
Merotec-XP is used for the treatment of infections caused by single or multiple bacteria originally sensitive to Meropenem.
Infections affecting the lungs (pneumonia)
Urinary Tract Infections
Infections in the abdomen
Infections that you can catch during or after the delivery
Infection of one or more of reproductive organs, including the uterus.
Skin infections
Infection in the blood (Septicemia)
Acute bacterial infection of the brain and its covering (Meningitis)
Infections caused by combinations of bacteria (Polymicrobial infections)
2. What you need to know before you take Merotec XP
Do not take Merotec XP
if you have an allergy or hypersensitivity to any ingredients of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to β-lactams in the past.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before taking Merotec XP.
• If you have health problems, such as liver or kidney problems.
• If you have had severe diarrhea after taking other antibiotics.
You may develop a positive test (Coombs test) which indicates the presence of antibodies that may destroy red blood cells. Your doctor will discuss this with you.
You may develop signs and symptoms of severe skin reactions (see section 4). If this happens talk to your doctor or nurse immediately so that they can treat the symptoms.
If you are not sure if any of the above applies to you, talk to your doctor or nurse before using Merotec XP.
Other medicines and Merotec XP
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
This is because Merotec XP can affect the way some medicines work and some medicines can have an effect on Merotec XP.
In particular, tell your doctor or nurse if you are taking any of the following medicines:
- Probenecid (used to treat gout).
- Valproic acid/sodium valproate/valpromide (used to treat epilepsy). Merotec XP should not be used because it may decrease the effect of sodium valproate.
- Oral anti-coagulant agent (used to treat or prevent blood clots).
Pregnancy, breast-feeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. It is preferable to avoid the use of Merotec XP during pregnancy. Your doctor will decide whether you should use Merotec XP. It is important that you tell your doctor if you are breastfeeding or if you intend to breastfeed before receiving Merotec XP. Small amounts of this medicine may pass into the breast milk. Therefore, your doctor will decide whether you should use Merotec XP while breastfeeding.
Driving and using machines
No studies on the effect on the ability to drive and use machines have been performed. Merotec XP has been associated with headache; tingling or pricking skin (paraesthesia). Any of these side effects could affect your ability to drive or operate machines. Merotec XP may cause involuntary muscle movements, leading the person's body to shake rapidly and uncontrollably (convulsions). This is usually accompanied with a loss of consciousness. Do not drive or use machines if you experience this side effect.
3. How to take Merotec XP
Always use this medicine exactly as your doctor or nurse has told you. Check with your doctor or nurse if you are not sure.
Use in Adults
- The dose depends on the type of infection that you have, where the infection is in the body, and how serious the infection is. Your doctor will decide on the dose that you need.
- The dose for adults is usually between 500 mg (milligrams) and 2 g (gram) of Meropenem. You will usually receive a dose every 8 hours. However, you may receive a dose less often if your kidneys do not work very well.
Use in children and adolescents
- The dose for children over 3 months old and up to 12 years of age is decided using the age and weight of the child. The usual dose is between 10 mg and 40 mg of Meropenem for each kilogram (kg) that the child weighs. A dose is usually given every 8 hours. Children who weigh over 50 kg will be given an adult dose.
How to use Merotec XP
• Merotec XP will be given to you as an I.V. bolus injection over approximately 5 minutes, or by I.V. infusion over approximately 15 to 30 minutes, using the specific available diluents.
• Your doctor or nurse will normally give Merotec XP to you.
• You should normally have your injections at the same times each day.
If you take more Merotec XP than you should
If you accidentally use more than your prescribed dose, contact your doctor or nearest hospital straight away.
If you forget to take Merotec XP
If you miss an injection, you should have it as soon as possible. However, if it is almost time for your next injection, skip the missed injection. Do not take a double dose (two injections at the same time) to make up for a forgotten dose.
If you stop taking Merotec XP
Do not stop having Merotec XP until your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Severe allergic reactions
If you have a severe allergic reaction, stop having Merotec XP and see a doctor straight away. You may need urgent medical treatment. The signs may include a sudden onset of:
Severe rash, itching or hives on the skin.
Swelling of the face, lips, tongue or other parts of the body.
Shortness of breath, wheezing or trouble breathing. Serious skin reactions which include
Serious hypersensitivity reactions involving fever, skin rash, and changes in the blood tests that check how the liver is working (increased levels of liver enzymes) and an increase in a type of white blood cell (eosinophilia) and enlarged lymph nodes. These may be signs of a multi-organ sensitivity disorder known as DRESS syndrome.
Severe red scaly rash, skin bumps that contain pus, blisters or peeling of skin, which may be associated with a high fever and joint pain.
Severe skin rashes that can appear as reddish circular patches often with central blisters on the trunk, skin peeling, ulcers of mouth, throat, nose, genitals, and eyes and can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome) or a more severe form (toxic epidermal necrolysis).
5. Damage to red blood cells (not known)
The signs include:
• Being breathless when you do not expect it.
• Red or brown urine.
If you notice any of the above, see a doctor straight away.
Other possible side effects:
Common (may affect up to 1 in 10 people)
• Abdominal (stomach) pain.
• Feeling sick (nausea).
• Being sick (vomiting).
• Diarrhoea.
• Headache.
• Skin rash, itchy skin.
• Pain and inflammation.
• Increased numbers of platelets in your blood (shown in a blood test).
• Changes in blood tests, including tests that show how well your liver is working. Uncommon (may affect up to 1 in 100 people)
• Changes in your blood. These include reduced numbers of platelets (which may make you bruise more easily), increased numbers of some white blood cells, decreased numbers of other white cells and increased amounts of a substance called ‘bilirubin’.
Your doctor may do blood tests from time to time.
• Changes in blood tests, including tests that show how well your kidneys are working.
• A tingling feeling (pins and needles)
. • Infections of the mouth or the vagina that are caused by a fungus (thrush).
• Inflammation of the bowel with diarrhea.
• Sore veins where Merotec XP is injected.
• Other changes in your blood. The symptoms include frequent infections, high temperature and sore throat. Your doctor may do blood tests from time to time.
Rare (may affect up to 1 in 1,000 people)
• Fits (convulsions).
• Acute disorientation and confusion (delirium)
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
6. Contents of the pack and other information
What Merotec XP contains
- The active substance is Meropenem and sulbactam.
Each vial contains:
Meropenem IP (Sterile) equivalent to Anhydrous Meropenem 1000 mg Sodium Carbonate (As buffer) equivalent to Sodium 90.2 mg Sulbactam Sodium (Sterile) equivalent to Sulbactam 500 mg Merotec XP is available in the following pack sizes: A vial of 1.5 gm with Sterile water for injection - 20 ml
7.0 Description
Meropenem: (4R, 5S, 6S)-3-[[(3S, 5S)-5- (Dimethyl carba moyl) -3-pyrrolidinyl] thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo [3.2.0] hept-2-ene-2-carboxylic acid.
Molecular Formula: C17H25N3O5S
Molecular Weight: 383.5 g/mol

Sulbactam Sodium: (2S, 5R)-3, 3-dimethyl-7-oxo-4-thia 1-azabicyclo [3.2.0] heptane-2-carboxylate 4, 4-dioxide.
Molecular Formula: C8H10NNaO5S
Molecular Weight: 255.22 g/mol

8.0 Pharmaceutical Properties
8.1 Incompatibilities
This medicinal product must not be mixed with other medicinal products except those mentioned in this section
Injection
Meropenem to be used for bolus intravenous injection should be reconstituted with sterile water for injection.
Infusion
For intravenous infusion, meropenem vials may be directly reconstituted with 0.9 % sodium chloride or 5% glucose solutions for infusion. Each vial is for single use only.
Standard aseptic techniques should be used for solution preparation and administration. The solution should be shaken before use.
Any unused product or waste material should be disposed of in accordance with local requirements.
8.2 Shelf-life
Refer on pack
After reconstitution:
Intravenous bolus injection administration
A solution for bolus injection is prepared by dissolving the drug product in water for injection to a final concentration of 50 mg/ml.
Chemical and physical in-use stability for a prepared solution for bolus injection has been demonstrated for 3 hours at up to 25° C or 12 hours under refrigerated conditions (2-8° C). From a microbiological point of view, unless the method of opening/reconstitution/dilution precludes the risk of microbiological contamination, the product should be used immediately. If not used immediately in-use storage times and conditions are the responsibility of the user.
Intravenous infusion administration
A solution for infusion is prepared by dissolving the drug product in either 0.9% sodium chloride solution for infusion or 5% dextrose solution for infusion to a final concentration of 1 to 20 mg/ml.
Chemical and physical in-use stability for a prepared solution for infusion using 0.9% sodium chloride solution has been demonstrated for 3 hours at up to 25° C or 24 hours under refrigerated conditions (2-8° C).
From a microbiological point of view, unless the method of opening/reconstitution/dilution precludes the risk of microbiological contamination, the product should be used immediately. If not used immediately in-use storage times and conditions are the responsibility of the user. Reconstituted solution of the product in 5%dextrose solution should be used immediately. The reconstituted solutions should not be frozen.
8.3 Packaging information
A vial of 1.5 gm with SWFI IP 20 ml.
8.4 Storage and handing instructions
Store in cool & dry place. Protect from light. Keep out of reach of children. After reconstitution, do not use in case any foreign particulate matter is observed inside the vial. Any unused portion must be discarded.
9.0 Patient Counselling Information
Serious Allergic Reactions
Advise patients that allergic reactions, including serious allergic reactions, could occur and that serious reactions require immediate treatment. Ask patient about any previous hypersensitivity reactions to Merotec-XP (meropenem and vaborbactam), penicillins, cephalosporins, other beta-lactams, or other allergens.
Seizures
Patients receiving Merotec-XP on an outpatient basis must be alerted of adverse events such as seizures, delirium, headaches and/or paresthesias that could interfere with mental alertness and/or cause motor impairment. Until it is reasonably well established that Merotec-XP is well tolerated, patients should not operate machinery or motorized vehicles.
Potentially Serious Diarrhea
Counsel patients that diarrhea is a common problem caused by antibacterial drugs including Merotec-XP, which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
Interaction with Valproic Acid
Counsel patients to inform their physician if they are taking valproic acid or divalproex sodium. Valproic acid concentrations in the blood may drop below the therapeutic range upon co-administration with Merotec-XP. If treatment with Merotec-XP is necessary and continued, alternative or supplemental anti-convulsant medication to prevent and/or treat seizures may be needed.
Interaction with Hormonal Contraceptives
Advise patients that administration of Merotec-XP may reduce the efficacy of hormonal contraceptives. Instruct patients to use effective alternative or back-up methods of contraception (such as condoms and spermicides) during treatment with Merotec-XP.
Antibacterial Resistance
Counsel patients that antibacterial drugs, including Merotec-XP, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Merotec-XP is prescribed to treat a bacterial infection, tell patients that although it is common to feel better early in the course of therapy, take the medication exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Merotec-XP or other antibacterial drugs in the future.
12.0 Date of revision of the text
25th September 2024