MethyPreg Tablets
Therapy Area
Pain management
Composition
Each modified release uncoated bilayered
tablet contains :
Pregabalin IP 75 mg
(As prolonged release)
Methylcobalamin IP 1500 mcg
(As immediate release)
Excipients q.s.
Appropriate overages of Vitamin added.
Description
MethyPreg is the combination of sustained release Pregabalin with Methylcobalamin. Pregabalin is a 3-substituted analogue of gamma-amino butyric acid (GABA) with an empirical formula of C H NO and 8 17 2 molecular weight of 159.2. Methylcobalamin is the active form of vitamin B with an empirical formula of C H CoN O P and molecular weight of 1344.4.
Pharmacology
Pharmacodynamics
Pregabalin binds with high affinity to α2δ subunits of voltage activated calcium channels, blocks Ca influx into nerve terminals, and decreases neurotransmitter release. This reduction in the transmitter release is probably responsible for reduction in transmission of pain impulses by decreasing release of excitatory neurotransmitters glutamate, adrenaline and substance P. Pregabalin also enhances the activity of glutamic acid decarboxylase, an enzyme that converts excitatory neurotransmitter glutamate to inhibitory neurotransmitter GABA (gamma amino butyric acid) in a single step. Methylcobalamin is the neurologically active form of B ; it is the methyl donor in DNA metabolism, which is needed to up-regulate gene transcription for peripheral nerve repair and regeneration. Additionally, 12 Methylcobalamin enhances protein metabolism in Schwann cells which are supporting cells of peripheral nervous system and also form myelin sheath around the nerves.
Pharmacokinetics
Pregabalin is rapidly absorbed with peak plasma concentrations occurring within 1.5 hours following both single and multiple dose administration. Oral bioavailability of Pregabalin is > 90% and is independent of dose. Cmax and AUC values increase proportionally following single and multiple dose administration. The rate of absorption of Pregabalin is decreased when given with food, but there is no clinically relevant effect on the total absorption. In humans, the apparent volume of distribution of Pregabalin following oral administration is approximately 0.5 L/kg. Pregabalin is not bound to plasma proteins. Pregabalin undergoes negligible metabolism in humans. Approximately 98% of the drug is excreted unchanged. Pregabalin is eliminated from the systemic circulation primarily by renal excretion as unchanged drug. Mean t½of Pregabalin is 6.3 hours. Its elimination is proportional to creatinine clearance. Pregabalin clearance is reduced in patients with impaired renal function. Methylcobalamin binds to intrinsic factor, and then is actively absorbed from the gastrointestinal tract. Methylcobalamin is extensively bound to specific plasma proteins called transcobalamins; transcobalamin II appears to be involved in the rapid transport of the cobalamins to tissues. Methylcobalamin is stored in the liver, excreted in the bile, and undergoes extensive enterohepatic recycling; part of a dose is excreted in the urine, most of it in the first 8 hours.
Indication
MethyPreg is indicated for
• For the treatment of adult patients with peripheral neuropathy
Dosage and Administration
The dose range is 150 to 600 mg per day given in either two or three divided doses.
The treatment can be started at a dose of 150 mg per day given as two or three divided doses. Based on individual patient response and tolerability, the dose may be increased to 300 mg per day after an interval of 3 to 7 days, and if needed, to a maximum dose of 600 mg per day after an additional 7-day interval.
Renal impairment
Table 1 : Pregabalin Dose Adjustment Based on Renal Function
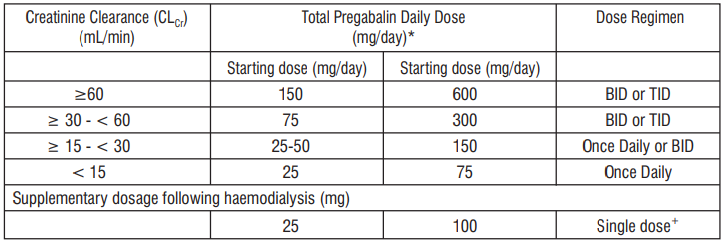
TID = Three divided doses
BID = Two divided doses
* Total daily dose (mg/day) should be divided as indicated by dose regimen to provide mg/dose
+ Supplementary dose is a single additional dose
Hepatic Impairment
No dose adjustment is required for patients with hepatic impairment.
Paediatric Population
The safety and efficacy of Pregabalin in children below the age of 12 years and in adolescents (12-17 years of age) have not been established.
Elderly
Elderly patients may require a dose reduction of Pregabalin due to a decreased renal function
Method of administration
Methypreg may be taken with or without food.
Contraindications
This combination is contraindicated in patients who are hypersensitive to Pregabalin, Methylcobalamin or to any ingredient in the formulation. It is also contraindicated in patients with Leber’s disease and Tobacco amblyopia.
Warnings & Precautions
Caution must be advocated in heart failure and diabetic patients in view of possibility of edema and weight gain induced by Pregabalin. Diabetic patients taking Pregabalin may develop skin ulcerations. This combination should not be discontinued abruptly due to the possibility of increasing seizure frequency, insomnia, nausea or diarrhea caused by Pregabalin, the anti-epileptic component of the combination. If it is to be stopped, it must be done gradually over 1 week.
Since Pregabalin can cause dizziness, somnolence and other manifestations of central nervous system like depression, patients should be advised neither to drive nor to operate complex machinery until they have gained sufficient experience on the combination to gauge whether or not it affects their mental and / or motor performance adversely.
If vision disturbances occur during Pregabalin therapy, medical advice must be sought. Also unexplained muscle pain, tenderness, or weakness, especially accompanied by malaise or fever requires prompt medical attention.
It is not to be taken along with alcohol, or be combined with other central nervous depressants.
Special Population
Women of childbearing potential/Contraception in males and females.
As the potential risk for humans is unknown, effective contraception must be used in women of child bearing potential.
Pregnancy
There are no adequate data from the use of Pregabalin in pregnant women. Pregabalin should not be used during pregnancy unless clearly necessary (if the benefit to the mother clearly outweighs the potential risk to the foetus).
Breast-feeding
Pregabalin is excreted into human milk. The effect of Pregabalin on newborns/infants is unknown. A decision must be made whether to discontinue breast-feeding or to discontinue Pregabalin therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman
Fertility
There are no clinical data on the effects of Pregabalin on female fertility
Adverse Reactions
The common adverse effects of Pregabalin given alone or in combination with other anti-epileptic agents are dizziness, somnolence, dry mouth, edema, blurred vision, weight gain, and "thinking abnormal" (primarily difficulty with concentration / attention). Asthenia, ataxia, abnormal gait, paresthesias, diplopia, blurred vision, visual field defect, nystagmus, nausea, tremor, vertigo, headache, nervousness, confusion and edema are other significant side effects of Pregabalin. Methylcobalamin is relatively safe and devoid of side effects.
Drug Interactions
Lorazepam, Ethanol and other CNS depressants
Pregabalin may potentiate the effects of these drugs and lead to additive CNS depression.
Phenytoin, phenobarbital, and primidone
These drugs have been associated with reduced vitamin B12 absorption and reduced serum and cerebrospinal fluid levels in some patients
Oxycodone
Additive cognitive impairment and gross motor function.
Thiazolidinedione antidiabetic agents
Higher frequencies of weight gain and peripheral edema were observed in patients taking both Pregabalin and a thiazolidinedione antidiabetic agent compared to patients taking either drug alone.
Histamine-2 receptor antagonists and PPI’s
Reduced secretion of gastric acid and pepsin produced by H2-blockers and PPI’s can reduce absorption of Methylcobalamin.
Colchicine
Co-administration with colchicines reduces Methylcobalamin absorption as colchicines disrupts normal mucosa and causes malabsorption
Overdosage
There is limited experience with overdose of Pregabalin. The highest reported accidental overdose of Pregabalin during the clinical development program was 8000 mg, and there were no notable clinical consequences. There is no specific antidote for overdose with Pregabalin. If indicated, elimination of unabsorbed drug may be attempted by emesis or gastric lavage; usual precautions should be observed to maintain the airway. General supportive care of the patient is indicated including monitoring of vital signs and observation of the clinical status of the patient.
Toxicity due to overdosage with Methylcobalamin is not known.
Storage
Store below 30°C. Protect from light and moisture.
Keep out of reach of children