Myotop DSR Tablets
Therapy Area
Pain management
1.0 Generic name
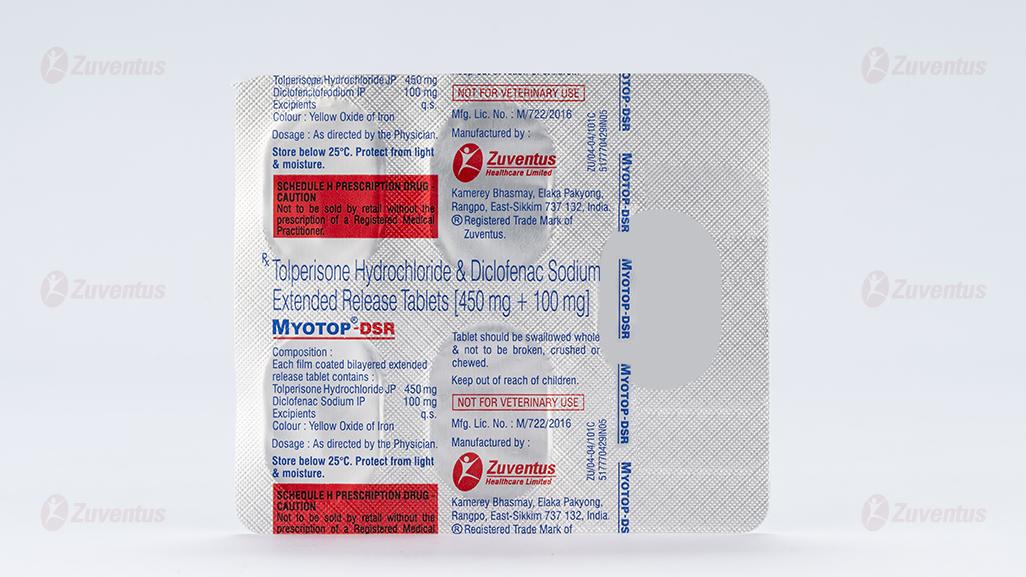
Tolperisone Hydrochloride & Diclofenac Sodium Extended Release Tablets (450 mg + 100 mg)
2.0 Qualitative and quantitative composition
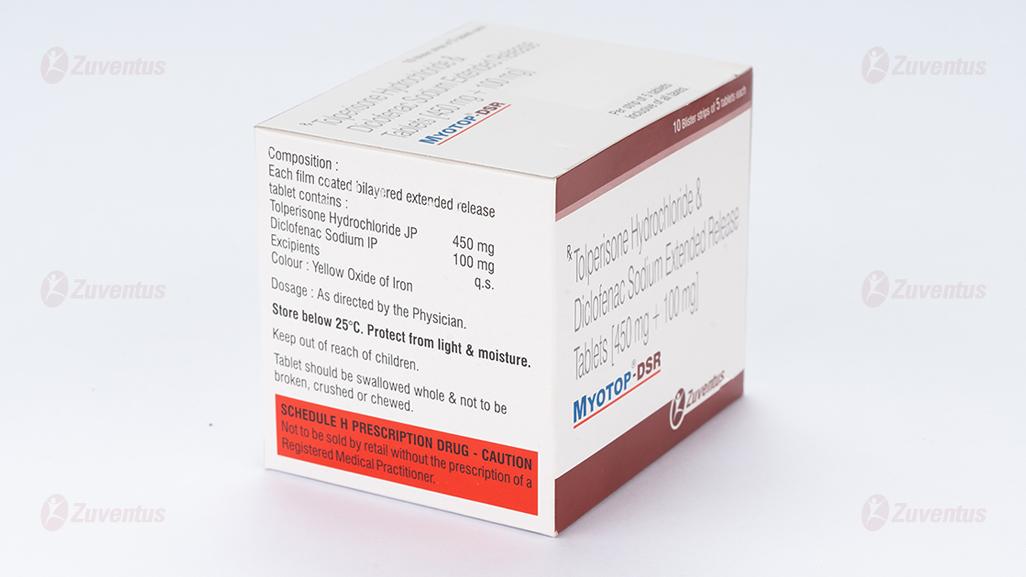
Each film coated bilayered extended release tablet contains
Tolperisone Hydrochloride 450 mg
Diclofenac Sodium IP 100 mg
Excipients q.s
Colour: Yellow Oxide of Iron
3.0 Dosage form and strength
Film coated bilayered extended release tablet
Tolperisone 450 mg + Diclofenac (100 mg) per tablet
4.0 Clinical particulars
4.1 Therapeutic Indication
For the treatment of Patients with acute muscle/musculoskeletal spasm in adult
4.2 Posology and method of administration
In adults, the recommended dosage is one tablet of Myotop-DSR once daily.
Method of administration: Oral
4.3 Contraindications
- Myotop-DSR is contraindicated in patients with history of hypersensitivity to any component of the product
- In patients suffering from myasthenia gravis
- Pregnancy and lactation
4.4 Special warnings and precautions for use
Precautions
- Close monitoring of renal parameters in patients with advanced renal disease is advisable.
- Myotop-DSR should be used with caution in patients with peptic ulcer disease, gastrointestinal perforation or bleeding, impaired renal or liver functions, blood dyscrasias, and hypersensitivity to aspirin or other NSAIDs.
- Caution should be exercised when administering this combination in patients chronically treated with NSAIDs.
- Precautions have to be taken when Myotop-DSR is used together with other muscle relaxants.
- Undesirable effects such as dizziness, drowsiness, fatigue and visual disturbances are possible.
Warning
- Rarely, hypersensitivity reactions can occur (pruritis, erythema, exanthema, dyspnea, angioneurotic edema and in single cases anaphylactic shock).
4.5 Drugs interactions
- Concomitant use of Myotop DSR with aspirin, methotrexate, digoxin, cyclosporin, ACE inhibitors, diuretics, lithium and warfarin is not advocated due to possibility of drug interactions with diclofenac.
- Tolperisone (in Myotop DSR) may enhance the effects of other neuromuscular blocking agents.
4.6 Use in special populations
Pregnancy and lactation
Myotop DSR should not be given during pregnancy and lactation
4.7 Effects on ability to drive and use machines
Undesirable effects such as dizziness, drowsiness, fatigue and visual disturbances are possible. If affected, patients should not drive or operate machinery.
4.8 Undesirable effects
Most common side effects include gastrointestinal (GI) upset with abdominal pain, heartburn, nausea, vomiting, diarrhea, dyspepsia, flatulence, GI ulcers, bleeding / perforation and dryness of mouth.
Other less common ADRs are abnormal renal function, edema, elevated liver enzymes, anemia, headaches, fatigue, muscle weakness or transient physical asthenia, dizziness, increased bleeding time, pruritus, rashes, arterial hypotension, hypersensitivity and other skin allergic reactions like skin rash, hives.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
4.9 Overdose
Symptoms:
Symptoms include headache, nausea, vomiting, epigastric pain, gastro-intestinal bleeding, diarrhea, disorientation, excitation, coma, drowsiness, dizziness, tinnitus, fainting, and occasionally convulsions. In cases of significant poisoning acute renal failure and liver damage are possible.
Therapeutic Measures: Patients should be treated symptomatically as required. Frequent or prolonged convulsions should be treated with intravenous diazepam. Activated charcoal/gastric lavage should be considered within one hour of ingestion of a potentially toxic amount. Renal and liver function should be closely monitored. Patients should be observed for at least four hours after ingestion of potentially toxic amounts. Other measures may be indicated by the patient's clinical condition.
5.0 Pharmacological properties
5.1 Mechanism of Action
Being, centrally acting muscle relaxant, tolperisone acts at the level of spinal cord by blocking sodium channels and calcium channels.
Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities. The mechanism of action of Diclofenac, like that of other NSAIDs, is related to prostaglandin synthesis inhibition.
5.2 Pharmacodynamic properties
Tolperisone exerts its spinal reflex inhibitory action predominantly via a pre synaptic inhibition of the transmitter release from the primary afferent endings via a combined action on voltage-gated sodium and calcium channels. Tolperisone increases the blood supply to skeletal muscles; this action is noteworthy since a muscle contracture may compress the small blood vessels and induce ischemia leading to release of pain stimulating compounds. Tolperisone causes preferential antinociceptive activity against thermal stimulation that is likely to be attributed to its local anesthetic action. Tolperisone causes muscle relaxation by its action on central nervous system. It also leads to membrane stabilization & has analgesic activity. This muscle relaxation is dose dependant.
Diclofenac reduces inflammation and by extension reduces nociceptive pain and combats fever.
5.3 Pharmacokinetic properties
|
Parameters |
Tolperisone |
Diclofenac |
| Tmax | 3.8 hr | 5.3 hr |
| Plasma protein binding | 60-75% | 99.7% |
| Half-life (T1/2) | 6.5 hrs | 2.3 hrs |
| Metabolism | Mainly metabolised by p450-mediated CYP2D6 metabolic pathway into its active metabolite hydroxymethyl tolperisone. | Undergoes hepatic metabolism by CYP3A4 |
| Excretion | Mainly in urine | 60% in urine, 30% in faeces |
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Tolperisone
Tolperisone is a central myorelaxant with solid non-clinical pharmacological and toxicological background. The non-clinical data support the clinical use of tolperisone in the currently approved indication and posology.
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development.
Effects in non-clinical studies were observed only at exposures considered sufficiently in excess of the maximum human exposure indicating little relevance to clinical use. Embryo toxic variations were observed in rats at 500 mg/kg body weight and in rabbits at 250 mg/kg body weight oral doses. These doses are several times higher than the human dose-range.
Diclofenac
Carcinogenesis
Long-term carcinogenicity studies in rats given diclofenac sodium up to 2 mg/kg/day (approximately 0.2 times the maximum recommended human dose [MRHD] of Diclofenac Capsules based on body surface area [BSA] comparison) have revealed no significant increase in tumor incidence. A 2-year carcinogenicity study conducted in mice employing diclofenac sodium at doses up to 0.3 mg/kg/day (approximately 0.014 times the MRHD based on BSA comparison) in males and 1 mg/kg/day (approximately 0.04 times the MRHD based on BSA comparison) in females did not reveal any oncogenic potential.
Mutagenesis Diclofenac sodium did not show mutagenic activity in in vitro point mutation assays in mammalian (mouse lymphoma) and microbial (yeast, Ames) test systems and was nonmutagenic in several mammalians in vitro and in vivo tests, including dominant lethal and male germinal epithelial chromosomal aberration studies in Chinese hamsters.
Impairment of Fertility
Diclofenac sodium administered to male and female rats at 4 mg/kg/day (approximately 0.4 times the MRHD based on BSA comparison) did not affect fertility.
7.0 Description
Myotop-DSR is a fixed dose combination of a Tolperisone (centrally acting muscle relaxant) and Diclofenac (NSAID).
Tolperisone
Chemical Name: 2,4'-Dimethyl-3-piperidinopropiophenone monohydrochloride
Molecular Formula: C16H24ClNO
Molecular Weight: 281.82
Diclofenac
Chemical Name: 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid, monosodium salt.
Molecular Formula: C14H10Cl2NNaO2
Molecular Weight: 318.14
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on pack
8.3 Packaging information
A blister strip of 5 tablets.
8.4 Storage and handing instructions
9.0 Patient Counselling Information
- Myotop-DSR Tablet helps relieve pain and muscle spasm that may occur due to strains, sprains, and muscle injuries. It is usually used along with rest and physical therapy.
- Advice patients not to take this medicine, if they are allergic to tolperisone hydrochloride and diclofenac sodium. Signs of an allergic reaction include a rash, itching or shortness of breath.
- Tolperisone is contraindicated in patients suffering from myasthenia gravies.
- Pregnancy: Tell female patients MYOTOP®-DSR should not be given during pregnancy and lactation. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible.
- Tell the patients if they get any side effects, talk to the doctor. This includes any possible side effects not listed in this leaflet. Advice patients if they have any further questions, ask the doctor or pharmacist.
- Tablet should be swallowed whole & not to be broken, crushed or chewed. Take it with or after food or a glass of milk.
- Do not stop taking it suddenly without talking to your doctor if you've been on it for a long time.
- Take it with food to avoid an upset stomach.
12.0 Date of revision
24/06/2024
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
- What MYOTOP DSR is and what it is used for
- What you need to know before you take MYOTOP DSR
- How to take MYOTOP DSR
- Possible side effects
- How to store MYOTOP DSR
- Contents of the pack and other information
1. What MYOTOP DSR is and what it is used for
MYOTOP DSR is a fixed dose extended release tablet of a Tolperisone 450mg (centrally acting muscle relaxant) and Diclofenac 100mg (NSAID).
MYOTOP DSR is used for the treatment of acute muscle/musculoskeletal spasm in adults.
2. What you need to know before you take MYOTOP DSR Do not take MYOTOP DSR:
- If you are allergic to any of the ingredients of this medicine.
- In you are suffering from a disease called myasthenia gravis
- If you are pregnant or lactating woman
Warnings and precautions
Talk to your doctor, pharmacist or nurse before taking MYOTOP DSR.
Take special care with MYOTOP DSR
Tell your doctor before you take this medicine if you:
- If you have advanced renal disease
- If you have peptic ulcer disease, gastrointestinal perforation or bleeding
- If you have impaired renal or liver functions
- If you have blood dyscrasias
- If you have hypersensitivity to aspirin or other NSAIDs
- If you are chronically treated with NSAIDs
- If you are using other muscle relaxants
Tell your doctor immediately if during treatment you suffer from
hypersensitivity reactions (pruritus, erythema, exanthema, dyspnea, angioneurotic edema)
Other medicines and MYOTOP DSR
Tell your doctor if you are taking or have taken the following medications.
- Aspirin
- Immune-system suppressant: methotrexate, cyclosporine
- Medicines used to treat heart failure: digoxin
- Medicines used to treat high blood pressure- ACE inhibitors, diuretics
- Medicine used to treat mania: lithium Medicines that stop blood clotting, such as warfarin
- Neuromuscular blocking agents
Pregnancy, breast-feeding and fertility
You should not take MYOTOP DSR when you are pregnant.
You should not breast-feed when taking MYOTOP DSR.
Driving and using machines
Undesirable effects such as dizziness, drowsiness, fatigue and visual disturbances are possible. If affected, you should not drive or operate machinery.
3. How to take MYOTOP DSR
Adults
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure.
In adults, the recommended dose is one tablet of Myotop-DSR once daily. Swallow the tablet whole with some water.
Use in children and adolescents
Myotop DSR is not used to treat children and adolescents
If you take more Myotop DSR than you should
Tell your doctor or pharmacist immediately.
If you forget to take Myotop DSR
Take the forgotten tablet as soon as you remember. Take the next tablet 24 hours after this and continue taking your tablets every 24 hours. Do not take a double dose to make up for a forgotten tablet.
If you stop taking Myotop DSR
Unless your doctor instructs you to stop treatment, it is important to continue taking Myotop DSR. If you stop and your original symptoms come back, tell your doctor or pharmacist immediately.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them Tell your doctor, nurse or pharmacist immediately if you notice any of these side effects during your treatment with Myotop DSR:
Most common side effects include
- Gastrointestinal (GI) upset with abdominal pain
- Heartburn
- Nausea, vomiting
- Diarrhea
- Dyspepsia (persistent or recurrent pain or discomfort in the upper abdomen)
- Flatulence (passing gas)
- GI ulcers
- Bleeding / perforation and dryness of mouth
Other side effects include:
- Changes in some blood test results including those measuring your kidney and liver
- Abnormal renal function
- Edema
- Anemia (low red blood cell)
- Headaches
- Fatigue
- Muscle weakness or transient physical asthenia
- Dizziness
- Increased bleeding time
- Pruritus (Itching), rashes
- Arterial hypotension
- Hypersensitivity and other skin allergic reactions like skin rash, hives
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effects with the help of your treating physician.
5. How to store Myotop DSR
Keep this medicine out of the sight and reach of children.
Store in a cool and dry place.
Protected from light.
6. Contents of the pack and other information
What Myotop DSR contains
- Myotop-DSR is a fixed dose combination of a Tolperisone 450 mg (centrally acting muscle relaxant) and Diclofenac 100mg (NSAID).
© Zuventus Healthcare Ltd., 2020. All rights reserved.