Nukast 4 mg Syrup
Therapy Area
Respiratory
1.0 Name of the medicinal product
Montelukast Sodium & Levocetirizine Dihydrochloride Syrup
2.0 Qualitative and quantitative composition
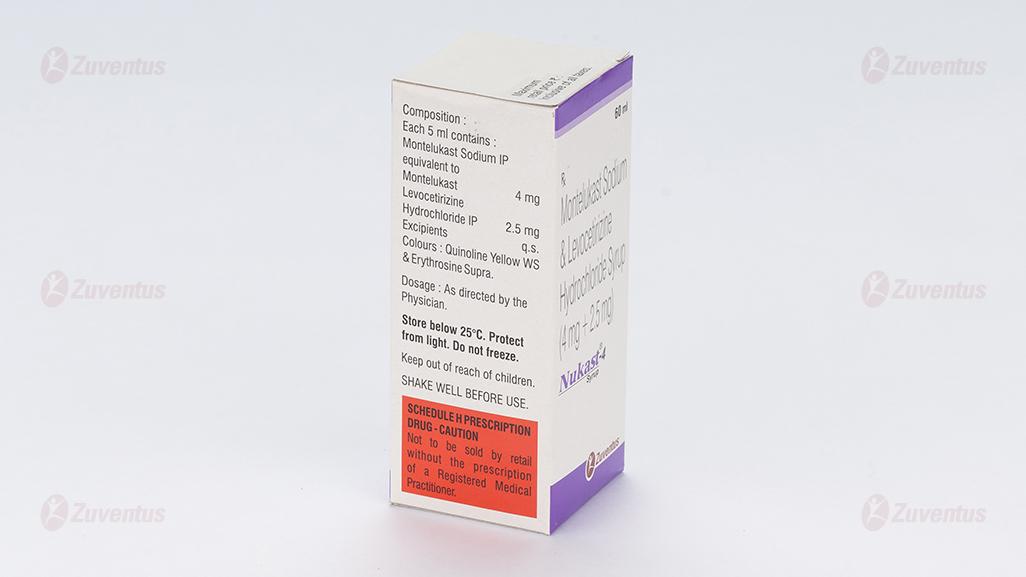
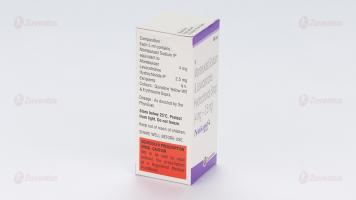
Each 5 ml contains: Montelukast Sodium USP Equivalent to Montelukast 4 mg Levocetirizine Dihydrochloride USP 2.5 mg
3.0 Dosage form and strength
Syrup
4.0 Clinical particulars
4.1 Therapeutic indication
For relief of symptoms of allergic rhinitis (seasonal and perennial).
4.2 Posology and method of administration
Children (2-5 years): 5 ml syrup as measured from the given cup once daily.
4.3 Contraindications
- known hypersensitivity to montelukast, levocetirizine, to other piperazine derivatives or to any of the excipients listed in the formulation.
- severe renal impairment at less than 10 ml/min creatinine clearance.
4.4 Special warnings and precautions for use
Montelukast
Eosinophilic Conditions
In rare cases, patients on therapy with montelukast may present with systemic eosinophilia sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition, which is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between, montelukast and those underlying conditions has not been established.
Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast.
Neuropsychiatric Events
Neuropsychiatric events have been reported in adult, adolescent, and pediatric patients taking montelukast. Post-marketing reports with montelukast use include agitation, aggressive behaviour or hostility, anxiousness, depression, disorientation, dream abnormalities, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thinking and behaviour (including suicide), and tremor. The clinical details of some post-marketing reports involving montelukast appear consistent with a drug-induced effect.
Patients and prescribers should be alert for neuropsychiatric events. Patients should be instructed to notify their prescriber if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with montelukast if such events occur.
Levocetirizine
Comparative clinical trials have revealed no evidence that levocetirizine at the recommended dose impairs mental alertness, reactivity or the ability to drive. In clinical trials the occurrence of somnolence, fatigue, and asthenia has been reported in some patients under therapy with levocetirizine. Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness and motor co-ordination such as operating machinery or driving a motor vehicle after ingestion of levocetirizine. Concurrent use of levocetirizine with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.
4.5 Drugs interactions
Montelukast
Montelukast may be administered with other therapies routinely used in the prophylaxis and chronic treatment of asthma. In drug-interactions studies, the recommended clinical dose of montelukast did not have clinically important effects on the pharmacokinetics of the following medicinal products: theophylline, prednisone, prednisolone, oral contraceptives (ethinyl estradiol/norethindrone 35/1), terfenadine, digoxin and warfarin.
The area under the plasma concentration curve (AUC) for montelukast was decreased approximately 40% in subjects with co-administration of phenobarbital, since montelukast is metabolised by CYP 3A4, caution should be exercised, particularly in children, when montelukast is co-administered with inducers of CYP 3A4, such as phenytoin, phenobarbital and rifampicin.
In vitro studies have shown that montelukast is a potent inhibitor of CYP 2C8. However, data from a clinical drug-drug interaction study involving montelukast and rosiglitazone (a probe substrate representative of medicinal products primarily metabolised by CYP 2C8) demonstrated that montelukast does not inhibit CYP 2C8 In vivo. Therefore, montelukast is not anticipated to markedly alter the metabolism of medicinal products metabolised by this enzyme (e.g paclitaxel, rosiglitazone and repaglinide.)
Levocetirizine
In vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes. No in vivo drug-drug interaction studies have been performed with levocetirizine.
Drug interaction studies have been performed with racemic cetirizine.
Pharmacokinetic interaction studies performed with racemic cetirizine demonstrated that cetirizine did not interact with antipyrine, pseudoephedrine, erythromycin, azithromycin, ketoconazole and cimetidine. There was a small decrease (~ 16%) in the clearance of cetirizine caused by a 400 mg dose of theophylline. It is possible that higher theophylline does could have a greater effect.
The extent of absorption of levocetirizine is not reduced with food although the rate of absorption is decreased.
Ritonavir increased the plasma AUC of cetirizine by about 42% accompanied by an increase in half-life (53%) and a decrease in clearance (29%) of cetirizine. The disposition of ritonavir was not altered by concomitant cetirizine administration.
In sensitive patients the simultaneous administration of cetirizine or levocetirizine and alcohol or other CNS depressants may have effects on the central nervous system, although it has been shown that the racemate cetirizine does not potentiate the effect of alcohol.
Renal Impairment
Levocetirizine is known to be substantially excreted by the kidneys and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Dosage adjustment may be required in patients with impaired renal function. Hence this combination is not recommended in patients with impaired renal function.
Hepatic Impairment
As levocetirizine is mainly excreted unchanged by the kidneys, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment. But montelukast is mainly excreted through bile; caution is to be exercised while prescribing this combination in patients with impaired hepatic function. The pharmacokinetics of montelukast in patients with more severe hepatic impairment or with hepatitis has not been evaluated.
4.6 Use in special populations (such as pregnant women, lactating women, paediatric patients, geriatric patients etc.)
Pregnancy
There are no adequate and well controlled studies of either montelukast or levocetirizine in pregnant women. Because animal reproduction studies are not always predictive of human response, this combination should be used during pregnancy only if it is considered to be clearly essential.
Lactation
It is not known if montelukast is excreted in human milk. Cetirizine has been reported to be excreted in human breast milk. Because levocetirizine is also expected to be excreted in human milk this combination is not recommended during lactation.
Pediatric Use
The safety and efficacy of montelukast in children with perennial allergic rhinitis below 6 months of age has not been established. The safety and effectiveness of levocetirizine in pediatric patients under 2 years of age have not been established.
Geriatric Use
Montelukast
No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Levocetirizine
Clinical studies of levocetirizine for each approved indication did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious usually star ting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
4.7 Effects on ability to drive and use machines
Montelukast
Montelukast is not expected to affect a patient's ability to drive a car or operate machinery. However, in very rare cases, individuals have reported drowsiness or dizziness.
Levocetirizine
Comparative clinical trials have revealed no evidence that levocetirizine at the recommended dose impairs mental alertness, reactivity or the ability to drive. Nevertheless, some patients could experience somnolence, fatigue and asthenia under therapy with levocetirizine.
Therefore, patients intending to drive, engage in potentially hazardous activities or operate machinery should take their response to the medicinal product into account.
4.8 Undesirable effects
There is no data available on undesirable effects of this combination. However, side effects have been reported with individual molecules.
Montelukast
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most common adverse reactions (incidence ≥ 5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.
Montelukast has been evaluated in 280 pediatric patients 2 to 14 years of age in a 2-week, multicentre, double-blind, placebo-controlled, parallel-group safety study. Montelukast
administered once daily in the evening had a safety profile similar to that of placebo. In this study, the following events occurred with a frequency ≥ 2% and at an incidence greater than placebo: headache, otitis media, pharyngitis, and upper respiratory infection.
The safety in patients 2 to 14 years of age with perennial allergic rhinitis is supported by the safety in patients 2 to 14 years of age with seasonal allergic rhinitis. The safety in patients 6 to 23 months of age is supported by data from pharmacokinetic and safety and efficacy studies in asthma in this pediatric population and from adult pharmacokinetic studies.
The following adverse reactions have been reported in post-marketing use:
Blood and lymphatic system disorders: Increased bleeding tendency.
Immune system disorders: Hypersensitivity reactions including anaphylaxis, hepatic eosinophilic infiltration.
Psychaitric disorders: Agitation including aggressive behaviour or hostility, anxiousness, depression, disorientation, dream abnormalities, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thinking and behaviour (including suicide), tremor.
Nervous system disorder: Drowsiness, Paresthesia /hypoesthesia, seizure.
Respiratory, thoracic and mediastinal disorders: Epistaxis
Cardiac disorders: Palpitations.
Gastro-intestinal disorders: Diarrhoea, dry mouth, dyspepsia, nausea, vomiting.
Hepatobiliary disorders: Elevated levels of serum transminases (ALT, AST), rare cases of cholestatic hepatitis, hepatocellular liver-injury, and mixed-pattern liver injury have been reported in patients treated with montelukast. Most of these occurred in combination with other confounding factors, such as use of other medications, or when montelukast was administered to patients who had underlying potential for liver disease, such as alcohol use or other forms of hepatitis.
Skin and subcutaneous tissue disorders : Angioedema, bruising, urticaria, pruritus, rash, erythema nodosum.
Musculoskeletal and connective tissue disorders : Arthralgia, myalgia including muscle cramps
General disorders and administration site conditions : Asthenia/fatigue, malaise, oedema.
Patients with asthma on therapy with montelukast may present with systemic eosinophilia. Sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction of oral cor ticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients.
Levocetirizine
Use of levocetirizine has been associated with somnolence, fatigue, nasopharyngitis, dry mouth and pharyngitis in subjects 12 years of age and older. Further uncommon incidences of adverse reactions like asthenia, abdominal pain, syncope and weight- increase were observed.
Adverse reactions reported in ≥ 2% of pediatric subjects aged 1-6 years exposed to levocetirizine 1.25 mg twice daily in a 2-week placebo-controlled clinical trial were pyrexia, diarrhea, vomiting and otitis media while in pediatric subjects 6-12 years of age exposed to levocetirizine 5 mg once daily in placebo-controlled clinical trials 4 and 6 weeks in duration were pyrexia, cough, somnolence, epitaxis. In pediatric subjects 6 months to 11 months receiving 1.25 mg levocetirizine once daily in a 2 week placebo controlled double blind safety trial the common side effects (≥ 3% subjects) were diarrhea and constipation.
In addition to the adverse reactions reported during clinical studies and listed above very rare cases of the following adverse drug reactions have been reported in post-marketing experience:
Immune system disorders: Hypersensitivity including anaphylaxis
Psychiatric disorders: Aggression, agitation, hallucination, depression
Nervous System disorders: Convulsion
Eyes disorders: Visual disturbances
Cardiac disorders: Palpitations, tachycardia.
Respiratory, Thoracic, and mediastinal disorders: Dyspnoea
Gastrointestinal disorders: Nausea, vomiting
Hepatobiliary disorders: Hepatitis Skin and subcutaneous tissue disorders: Angioneurotic edema, fixed drug eruption, pruritus, rash urticaria
Musculoskeletal, connective tissues, and bone disorders: Myalgia
Investigations: Weight Increased, abnormal liver functions tests
4.9 Overdose
There is no data reported on the overdosage of this combination. However, overdosage has been reported with individual molecules.
Montelukast
No specific information is available on the treatment of overdosage with montelukast. In chronic asthma studies, montelukast has been administered at doses up to 200 mg/day to adult patients for 22 weeks and, in short-term studies, up to 900 mg/day to patients for approximately a week without clinically important adverse experiences.
There have been reports of acute overdosage in post-marketing experience and clinical studies with montelukast. These include reports in adults and children with a dose as high as 100 mg. The clinical and laboratory findings observed were consistent with the safety profile in adults and pediatric patients. There were no adverse experiences in the majority of overdosage reports. The most frequently occurring adverse experiences were consistent with the safety profile of montelukast and included abdominal pain, somnolence, thirst, headache, vomiting and psychomotor hyperactivity.
In the event of overdose, it is reasonable to employ the usual supportive measures : e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive therapy, if required.
It is not known whether montelukast is removed by peritoneal dialysis or hemodialysis.
Levocetirizine
Symptoms of overdose may include drowsiness in adults and initially agitation and restlessness, followed by drowsiness in children. There is no known specific antidote to levocetirizine. Should overdose occur, symptomatic or supportive treatment is recommended. Gastric lavage should be considered following short-term ingestion. Levocetirizine is not effectively removed by haemodialysis.
5.0 Pharmacological properties
5.1 Pharmacodynamic properties
The cysteinyl leukotrienes (LTC4, LTD4, LTE4) are potent inflammatory eicosanoids released from various cells including mast cells and eosinophils. These important pro-asthmatic mediators bind to cysteinyl leokotriene (CysLT) receptors.
The CysLT type-1 (CysLT1) receptor is found in the human airway (including airway smooth muscle cells and airway macrophages) and on other pro-inflammatory cells (including eosinophils and certain myeloid stem cells). CysLTs have been correlated with the pathophysiology of asthma and allergic rhinitis.
In allergic rhinitis, CysLTs are released from the nasal mucosa after allergen exposure during both early and late-phase reactions and are associated with symptoms of allergic rhinitis. Intranasal challenge with CysLTs has been shown to increase nasal airway resistance and symptoms of nasal obstructions.
Montelukast is an orally active compound that binds with high affinity and selectivity to the CysLT1 receptor. Montelukast inhibits physiologic actions of LTD4 at the CysLT1 receptor without any agonist activity. In patients with seasonal allergic rhinitis aged 15 years and older who received montelukast, a mean increase of 0.2% in peripheral blood eosinophil counts was noted, compared with a mean increase of 12.5% in placebo-treated patients, over the double-blind treatment periods; this reflects a mean difference of 12.3% in favour of montelukast. The relationship between these observations and the clinical benefits of montelukast noted in the clinical trials is not known.
Levocetirizine
Levocetirizine, the R-enantiomer of cetirizine, is a potent and selective antagonist of peripheral H1-receptors. Binding studies revealed that levocetirizine has high affinity for human H1-receptors (Ki=3.2 nmol/l). Levocetirizine has an affinity 2-fold higher than that of cetirizine (Ki=6.3 nmol/1). Levocetirizine dissociates from H1 - receptors with a half-life of
115±38 min. After single administration, levocetirizine shows receptor occupancy of 90% at 4 hours and 57% at 24 hours.
The onset of action of levocetirizine 5 mg in controlling pollen-induced symptoms has been observed at 1 hour post drug intake in placebo controlled trials in the model of the allergen challenge chamber.
In vitro studies (Boyden chambers and cell layers techniques) show that levocetirizine inhibits eotaxin-induced eosinophil transendothelial migration through both dermal and lung cells. A pharmacodynamic experimental study in vivo (skin chamber technique) showed three main inhibitory effects of levocetirizine 5 mg in the first 6 hours of pollen-induced reaction compared with placebo in 14 adult patients: Inhibition of VCAM-1 release, modulation of vascular permeability and a decrease in eosinophil recruitment.
Pharmacodynamic studies in healthy volunteers demonstrate that at half the dose levocetirizine has comparable activity to cetirizine, both in the skin and in the nose.
A QT/QTc study using a single dose of 30 mg of levocetirizine did not demonstrate an effect on the QTc interval. While a single dose of levocetirizine had no effect, the effects of levocetirizine may not be at steady state following single dose. The effect of levocetirizine on the QTc interval following multiple dose administration is unknown. Levocetirizine is not expected to have QT/QTc effects because of the results of QTc studies with cetirizine and the long post-marketing history of cetirizine without reports of QT prolongation.
5.2 Pharmacokinetic properties
Montelukast
Absorption
Montelukast is rapidly absorbed following oral administration. For the 4 mg chewable tablet, the mean Cmax is achieved 2 hours after administration in pediatric patients 2 to 5 years of age in the fasted state.
The 4 mg oral granule formulation is bioequivalent to the 4 mg chewable tablet when administered to adults in the fasted state. The co-administration of the oral granule formulation with applesauce did not have a clinically significant effect on the pharmacokinetics of montelukast. A high fat meal in the morning did not affect the AUC of montelukast oral granules; however, the meal decreased Cmax by 35% and prolonged Tmax from 2.3 ± 1.0 hours to 6.4 ± 2.9 hours.
The safety and efficacy of montelukast in patients with seasonal allergic rhinitis were demonstrated in clinical trials in which the 10 mg film-coated tablet was administered in the morning or evening without regard to the time of food ingestion.
Distribution
Montelukast is more than 99% bound to plasma proteins. The steady state volume of distribution of montelukast averages 8 to 11 liters. Studies in rats with radiolabeled montelukast indicate minimal distribution across the blood-brain barrier.
In addition, concentrations of radiolabeled material at 24 hours post dose were minimal in all other tissues.
Metabolism
Montelukast is extensively metabolized. In studies with therapeutic doses, plasma concentrations of metabolites of montelukast are undetectable at steady state in adults and pediatric patients.
In vitro studies using human liver microsomes indicate that cytochromes P450 3A4 and 2C9 are involved in the metabolism of montelukast. Clinical studies investigating the effect of known inhibitors of cytochromes P450 3A4 (e.g ketoconazole, erythromycin) or 2C9 (e.g., fluconazole) on montelukast pharmacokinetics have not been conducted. Based on further in vitro results in human liver microsomes, therapeutic plasma concentrations of montelukast do not inhibit cytochromes P450 3A4, 2C9, 1A2, 2A6, 2C19 or 2D6. However, in vitro studies have shown that montelukast is a potent inhibitor of cytochrome P450 2C8; however, data from a clinical drug-drug interaction study involving montelukast and rosiglitazone (a probe substrate representative of drugs primarily metabolized by CYP 2C8) demonstrated that montelukast does not inhibit CYP 2C8 in vivo, and therefore is not anticipated to alter the metabolism of drugs metabolized by this enzyme.
Elimination
The plasma clearance of montelukast averages 45 mL/min in healthy adults. Following an oral dose of radiolabeled montelukast, 86% of the radioactivity was recovered in 5-day fecal collection and <0.2% was recovered in urine. Coupled with estimates of montelukast oral bioavailability, this indicates that montelukast and its metabolites are excreted almost exclusively via the bile.
In several studies, the mean plasma half-life of montelukast ranged from 2.7 to 5.5 hours in healthy young adults. The pharmacokinetics of montelukast is nearly linear for oral doses up to 50 mg. During once-daily dosing with 10 mg montelukast, there is little accumulation of the parent drug in plasma (14%).
Levocetirizine
The pharmacokinetics of levocetirizine is linear with dose and time independent with low inter-subject variability. The pharmacokinetic profile is the same when given as the single enantiomer or when given as cetirizine. No chiral inversion occurs during the process of absorption and elimination.
Absorption
Levocetirizine is rapidly and extensively absorbed following oral administration. Peak plasma concentrations are achieved 0.9 h after dosing. Steady state is achieved after two days. Peak concentrations are typically 270 ng/ml and 308 ng/ml following a single and a repeated 6 mg o.d. dose, respectively. The extent of absorption is dose-independent and is not altered by food, but the peak concentration is reduced and delayed.
Distribution
No tissue distribution data are available in humans, neither concerning the passage of levocetirizine through the blood-brain-barrier. Levocetirizine in 90% bound to plasma proteins. The distribution of levocetirizine is restrictive, as the volume of distribution is 0.4 l/kg.
Biotransformation
The extent of metabolism of levocetirizine in humans is less than 14% of the dose and therefore differences resulting from genetic polymorphism or concomitant intake of enzyme inhibitors are expected to be negligible. Metabolic pathways include aromatic oxidation, N and O-dealkylation and taurine conjugation. Dealkylation pathways are primarily mediated by CYP 3A4 while aromatic oxidation involved multiple and/or unidentified CYP isoforma. Levocetirizine had no effect on the activities of CYP isoenzymes 1A2, 2C0, 2C19, 2D8, 2E1 and 3A4 at concentrations well above peak concentrations achieved following a 6 mg oral dose.
Due to its low metabolism and absence of metabolic inhibition potential, the interaction of levocetirizine with other substances or vice-versa, is unlikely.
Elimination
The plasma half-life in adults is 7.9 ± 1.9 hours. The half-life is shorter in small children. The mean apparent total body clearance is 0.63 ml/min kg. The major route of excretion of levocetirizine and metabolites is via urine, accounting for a mean of 85.4% of the dose. Excretion via faeces accounts for only 12.9% of the dose. Levocetirizine is excreted both by glomerular filtration and active tubular secretion.
5.3 Preclinical safety data
Montelukast sodium was found not to be genotoxic. Montelukast sodium was negative in microbial and mammalian cell mutagenesis assays, with and without metabolic activation. There was no evidence of clastogenic activity in the in vitro chromosomal aberration assay in Chinese Hamster Ovary cells, with or without a microsomal enzyme activation system, or of DNA damage in the in vitro alkaline elution assay in rat hepatocytes. Similarly, there was no induction of chromosomal aberrations in bone marrow cells of male or female mice.
Montelukast sodium was not carcinogenic when administered at oral doses of up to 200 mg/kg/day in 104-week study in rats, nor at oral doses up to 100 mg/kg/day in a 91-week study in mice. Systemic exposure in these studies, in terms of the plasma AUC for parent drug, was at least 30 times higher than that in humans at recommended dose levels.
Levocetirizine
Levocetirizine is the R-enantiomer of the marketed cetirizine. The long term oral toxicity, fertility and early developmental and prenatal postnatal developmental toxicity studies with cetirizine represent the toxicity profile of levocetirizine with supplemental bridging toxicity, developmental and genotoxicity studies conducted with levocetirizine. In genotoxicity studies, cetirizine was negative in the Ames, Human Peripheral Lymphocytes Chromosomal Aberration, Mouse Lymphoma and Mouse Micronucleus assays. Levocetirizine, In the carcinogenicity studies in rodents, the dietary doses were 1, 4 and 16 mg/kg for the mouse and 3, 8 and 20 mg/kg for the rat. The rats showed liver toxicity (hypertrophy, vacuolation and fat deposit). No tumors were seen in rats that were clinically significant, while male mice showed benign liver tumors which were due to enzyme induction.
6.0 Pharmaceutical particulars
6.1 List of excipients
Methylparaben Sodium, Propylparaben Sodium, Polyoxyl 40 Hydrogenated Castor Oil, Glycerol, Saccharin Sodium, Mango Flavour RSV, Colour Quinoline Yellow WS, Colour Erythrosine Supra, Disodium Edetate, Sodium Hydroxide, Hydrochloric Acid
6.2 Incompatibilities
Not Applicable
6.3 Shelf life
24 MONTHS
6.4 Special precautions for storage
Protect from light.
6.5 Nature and contents of container Nukast Syrup:
1x60ml, PET Bottle 6.6Special precautions for disposal and other handling No special requirements for disposal. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
10.0 Date of revision of the text
Nov 2020
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
- What Nukast-4 Syrup is and what it is used for
- What you need to know before you take Nukast-4 Syrup
- How to take Nukast -4 Syrup
- Possible side effects
- How to store Nukast -4 Syrup
- Contents of the pack and other information
1. What Nukast -4 Syrup is and what it is used for
What Nukast -4 Syrup is
Nukast is combination of Montelukast and Levocetirizine. Montelukast is a leukotriene receptor antagonist that blocks substances called leukotrienes. Levocetirizine is an anti-allergic medication. Nukast-4 Syrup is used in children for the treatment of signs of illness (symptoms) associated with allergic rhinitis (including persistent allergic rhinitis)
2. What you need to know before you take Nukast
Tell your doctor about any medical problems or allergies you have now or have had.
Do not take Nukast
If you are allergic to montelukast/ levocetrizine or any of the other ingredients of this medicine. Severe renal impairment (Creatinine clearance less than 10 ml/min)
Warnings and precautions
Talk to your doctor or pharmacist before taking Nukast.
Montelukast
Acute Asthma: Montelukast is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmatics. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled β2-agonist.
Concomitant Corticosteroid Use: While the dose of inhaled corticosteroid may be reduced gradually under medical supervision, montelukast should not be abruptly substituted for inhaled or oral corticosteroids.
Aspirin Sensitivity: Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast.
Eosinophilic Conditions: Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients.
Neuropsychiatric Events: Prescribers should carefully evaluate the risks and benefits of continuing treatment with montelukast if such events occur.
Phenylketonuria: Phenylketonuric patients should be informed about the presence of phenylalanine (a component of aspartame) in this product.
Levocetirizine
Somnolence: Concurrent use of levocetirizine with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.
Urinary Retention: Levocetirizine should be used with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as levocetirizine may increase the risk of urinary retention. Discontinue, if urinary retention occurs.
Pediatric
The safety and efficacy of levocetirizine in pediatric patients under 2 years of age have not been established.
Pregnancy
No clinical studies available in human pregnancy. Therefore, NUKAST syrup should not be used in pregnancy.
Lactation
It is not known if montelukast is excreted in human milk. Cetirizine has been reported to be excreted in human breast milk. Because levocetirizine is also expected to be excreted in human milk this combination is not recommended during lactation.
Hepatic insufficiency
As levocetirizine is mainly excreted unchanged by the kidneys, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment. But montelukast is mainly excreted through bile; caution is to be exercised while prescribing this combination in patients with impaired hepatic function. The pharmacokinetics of montelukast in patients with more severe hepatic impairment or with hepatitis has not been evaluated.
Renal impairment
Levocetirizine is known to be substantially excreted by the kidneys and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Dosage adjustment may be required in patients with impaired renal function. Hence this combination is not recommended in patients with impaired renal function.
Other medicines and Nukast
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines including those obtained without a prescription.
Some medicines may affect how Nukast works, or Nukast may affect how other medicines work.
Tell your doctor if you are taking the following medicines before starting Montelukast:
- Phenobarbital (used for treatment of epilepsy)
- Phenytoin (used for treatment of epilepsy)
- Rifampicin (used to treat tuberculosis and some other infections)
- Gemfibrozil (used for treatment of high lipid levels in plasma)
Taking Nukast with food and drink
Nukast-4 syrup may be taken with or without food.
Driving and using machines
Nukast-4 syrup has no or negligible influence on the ability to drive and use machines. However, individuals have reported drowsiness or dizziness. Therefore, patients intending to drive, engage in potentially hazardous activities or operate machinery should take their response to the medicinal product into account.
3. How to take Nukast
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
- You should take only one tablet of Nukast once a day as prescribed by your doctor.
- It should be taken even when you have no symptoms or have an acute asthma attack.
For Children (2-5 years): 5 ml syrup as measured from the given cup once daily.
Nukast-4 syrup can be taken with or without food.
Hepatic Impairment: No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency.
If you are taking Nukast-4 syrup, be sure that you do not take any other products that contain the same active ingredient, montelukast and levocetirizine.
This medicine is for oral use.
You can take Nukast-4 syrup with or without food.
If you take more Nukast-4 syrup than you should
Contact your doctor immediately for advice.
There were no side effects reported in the majority of overdose reports. The most frequently occurring symptoms reported with overdose in adults and children included abdominal pain, somnolence, thirst, headache, vomiting and psychomotor hyperactivity.
In the event of overdose, it is reasonable to employ the usual supportive measures; e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive therapy, if required. Gastric lavage should be considered following short-term ingestion of Nukast.
If you forget to take Nukast-4 syrup
Try to take Nukast-4 syrup as prescribed. However, if you miss a dose, just resume the usual schedule of 5 ml syrup as measured from the given cup once daily.
Do not take a double dose to make up for a forgotten dose.
If you stop taking Nukast
Nukast-4 syrup can treat your rhinitis (inflammation of nasal mucosa) only if you continue to take it. It is important to continue taking Nukast for as long as your doctor prescribes. It will help control your rhinitis.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Serious side effects
Talk with your doctor immediately if you notice any of the following side effects, which may be serious, and for which you may need urgent medical treatment.
Pediatric Patients: 2 to 5 Years of Age (≥ 2%):
Abdominal pain, diarrhea, headache, rhinorrhea, sinusitis, otitis, influenza, rash, ear pain, gastroenteritis, eczema, urticaria, varicella, pneumonia, dermatitis, and conjunctivitis, thirst.
Levocetirizine:
Use of levocetirizine has been associated with somnolence, fatigue, asthenia, and urinary retention.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store Nukast-4 syrup?
Keep this medicine out of the sight and reach of children. Store below 30°C. Protect from light. Do not freeze.
Do not use this medicine after the expiry date which is stated on the blister after EXP. The expiry date refers to the last date of that month.
This medicinal product does not require any special storage conditions.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Nukast-4 Syrup contains:
The active substance is montelukast and levocetirizine.
Each uncoated bilayered tablet contains Montelukast sodium equivalent to Montelukast 4 mg
Levocetirizine hydrochloride 2.5 mg Excipients q.s.