Oxyptadil Injection
Therapy Area
Vasoactive Intestinal Peptide
1.0 Generic name
Aviptadil Injection 150 µg/10 ml
2.0 Qualitative and quantitative composition
Each ml contains :
Aviptadil 15 µg
Water for Injections IP q.s.
3.0 Dosage form and strength
Injection 150 µg / 10 ml
4.0 Clinical particulars
4.1 Therapeutic indication
For treatment of patients with severe COVID-19 with Acute Respiratory Distress Syndrome (ARDS).
4.2 Posology and method of administration
Aviptadil intravenous infusion is administered by infusion pump in escalating doses for 3 successive days
- Day 1 : Aviptadil 0.166 mcg/kg/hr (equivalent to 1 vial of Aviptadil Injection)
- Day 2 : Aviptadil 0.332 mcg/kg/hr (equivalent to 2 vials of Aviptadil Injection)
- Day 3 : Aviptadil 0.498 mcg/kg/hr (equivalent to 3 vials of Aviptadil Injection)
Duration of infusion depends on the patient's body weight
- Body weight < 60 kg - 14 hour infusions of Aviptadil at escalating doses on 3 successive days.
- Body weight 60 - 90 kg - 12 hour infusions of Aviptadil at escalating doses on 3 successive days.
- Body weight > 90 kg - 10 hour infusions of Aviptadil at escalating doses on 3 successive days.
4.3 Contraindications
Aviptadil is contraindicated in patients who are hypersensitive to any component of this product.
4.4 Special warnings and precautions for use
Mild transient flushing of the face or trunk occurs commonly. This is rarely associated with discomfort and palpitations or tachycardia in which cases patients may be withdrawn from treatment. Aviptadil must be used with caution in patients with severe cardiovascular or cerebrovascular conditions.
4.5 Drugs interactions
No clinically relevant interaction was observed concomitantly to anti-hypertensive drugs or other cardiovascular drugs.
4.6 Use in special populations
No specific studies in special patient populations have been performed so far
4.7 Effects on ability to drive and use machines
No trials on the effect on the ability to drive a car or use machines have been carried out
4.8 Undesirable effects
Gastrointestinal Disorders e.g. Diarrhea, Vascular disorders - Hypotension, cutaneous flushing, facial flushing and Infusion related reactions.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
4.9 Overdose
No case of overdose has been reported
5.0 Pharmacological properties
5.1 Mechanism of action
Aviptadil is a synthetic form of Human Vasoactive Intestinal Polypeptide (VIP). VIP is highly concentrated in the lung and it blocks apoptosis, caspase-3 activation in the lung, inhibits inflammatory cytokines like IL6 and TNF alpha production and reverses CD4/CD8 ratio. VIP increases surfactant production by up regulation of choline phosphate cytidylyltransferase, which increase the incorporation of methyl choline to phosphatidylcholine, the major components of pulmonary surfactant. It also up regulate C Fos protein expression in type II alveolar cells, which increase the synthesis of pulmonary surfactant phospholipids and induce surfactant protein A expression. VIP reduces ischemia-reperfusion injury in animal models. It reduces cell death by inhibiting activation induced perforin, granzyme B and caspase activity. VIP reduces pulmonary inflammation by reducing the production of pro inflammatory cytokines. It inhibits the synthesis and activation of NF-kB, which block the production of TNF alpha. In addition to that VIP blocks SARS-CoV-2 replication in the lungs and monocyte. VIP and pituitary adenylate cyclase activating polypeptide (PACAP) inhibit SARS CoV-2 RNA synthesis in human lung epithelial cell (by 41%) and human primary monocytes (by 33-45%). It also blocks viral cytopathic effect demonstrated by reduced LDH release (by 40%). SARS CoV-2 attack mainly type II cells and results in the death of alveolar type II (AT 11) cells which produces surfactant, resulting in profound defect in oxygenation, leading to hypoxia. Aviptadil a synthetic form of VIP results in rapid clinical recovery in patients with SARS-CoV-2 infection.
5.2 Pharmacodynamic properties
Pulmonary circulation
After intravenous infusion of Aviptadil an increase of heart rate, stroke volume and cardiac output was reported. Right atrial and pulmonary wedge pressure remained unchanged while pulmonary vascular resistance significantly decreased as well as pulmonary arterial systolic pressure. Aviptadil vasodilating properties are 50 times more potent than prostacyclin and independent of the endothelium.
Immunotolerogenic response
In Pulmonary Sarcoidosis, the presumed primary therapeutic mechanism of action of inhaled Aviptadil is a combination of anti-inflammatory properties and induction of tolerogenic immune response of immune cells localized in the lungs.
Systemic circulation
In healthy volunteers intravenous Aviptadil reduced systemic vascular resistance due to its potent vasodilatory effects, followed by increase of heart rate and decrease of blood pressure.
Airway responses
During intravenous infusion (20 ng Aviptadil /kg/min, 30 min), ventilation - perfusion relationships of the lungs (VA/Q) were significantly changed. VA/Q distributions determined by inert gas elimination technique
were shifted to lower values but arterial oxygen tension remained unchanged. Therefore, Aviptadil alters the ventilation - perfusion distributions but generates no shunt and does not cause hypoxia.
5.3 Pharmacokinetic properties
Absorption
Studies with radiolabeled Aviptadil (single intravenous bolus injection of 300 pmol [1 µg]) demonstrate that after the initial rapid clearance from the circulation, the lung is the primary site of peptide binding. Within 30 min., about 45% of the radioactivity is found in the lungs. Only minimal activity was found in the gastrointestinal tract, and almost no activity was seen in normal liver or spleen during the observation period of 24 hours. The radioactivity in the lungs decreased at 4 hours to 25%, and after 24 hours to 10%. Radioactivity was excreted into the urine (35% of the injected dose after 4 hours, and 90% after 24 hours). Half-life of Aviptadil in blood was 1 minute, the metabolic clearance rate was 9 ml/kg/min and the apparent volume of distribution for Aviptadil was about 14 ml/kg.
Distribution
Aviptadil binding to its receptors occurs in discrete locations within the gastrointestinal, respiratory, and genital tracts. Aviptadil is localized on respiratory epithelium, smooth muscles of the airways, blood vessels and alveolar walls.
Elimination
After injection of 1 µg radioactively labelled Aviptadil as bolus to patients a very rapid tissue distribution was observed. Within 30 minutes about 45% of the radioactivity was found in the lungs. Over an observation period of 24 hours only minimal activity was detected in the gastrointestinal tract and almost no activity was found in the liver or spleen. Radioactivity in the lungs decreased within four hours to 25% and within 24 hours to 10%. The half-life of Aviptadil in plasma is about 1-2 minutes. After injection of radiolabelled Aviptadil radioactivity was almost completely eliminated by the kidneys, 35% within 4 hours, and 90% within 24 hours.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
In a battery of toxicological tests including repeated dose toxicity in rats and dogs, cardiopulmonary tests in dogs and functional behavioural assays in rats, no adverse side effects were observed with NAP concentrations that were ∼ 500-fold higher than the biologically active dose.
Effect of aviptadil on fertility and early embryonal development
• Aviptadil infusions in rabbits can increase plasma progesterone from both the basal levels of estrus and from the peak levels preceding ovulation.
• Infusions of Aviptadil at the time of the preovulatory steroid surge or during ovulation have little effect upon fertility or gamete transport in the rabbit.
• Endogenous VIP may play a role in the regulation of progesterone secretion in the rabbit.
Effect of aviptadil on embryogenesis
In rats, Aviptadil binding sites in the embryo were analysed during embryonic days E10-E13. Aviptadil binding was almost exclusively located to the brain and spinal cord. The investigation of expression of Aviptadil mRNA from embryo in this stage (E11) revealed totally negative results, indicating extraembryonic, maternal Aviptadil supply to the embryo. Indeed, beginning at day E9, there is an enormous increase of Aviptadil concentration in maternal blood, 6-10 times greater than later on during pregnancy. Data indicate that Aviptadil from maternal tissues may be the source of Aviptadil acting on the embryonic tissues, transferred to the embryo undegraded. On day E17, Aviptadil mRNA expression was easily detectable all over the embryo, and maternal Aviptadil concentration in the blood decreased to very low levels. Aviptadil is required for embryonic development.
Genotoxicity
No evidence of genotoxic potential has been found in in vitro and in vivo studies.
7.0 Description
Non-proprietary name : Aviptadil
Other name : VIP (Vasoactive Intestinal Peptide)
Structure of Aviptadil
Aviptadil (VIP) is a linear, 28-AA peptide with an amidated C-terminus. All amino acids of Aviptadil are in Lconfiguration.
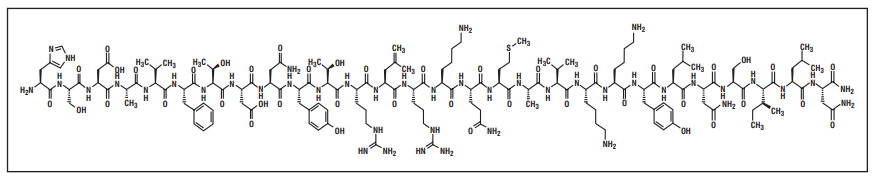
Chemical name : H-His-Ser-Asp-Ala-Val-Phe-Thr-Asp-Asn-Tyr-Thr-Arg-Leu-Arg-Lys-Gln-Met-Ala-ValLys-Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn-NH2
Molecular formula : C147H238N44O42S
Molecular weight : 3325.8 g/mol
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
A vial of 10 ml.
8.4 Storage and handing instructions
Store in a refrigerator (2°C - 8°C). Do not freeze.
Keep out of reach of children.
Do not use in case any foreign particulate matter is observed inside the vial.
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you:
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Oxyptadil® is and what it is used for
2. What you need to know before you take Oxyptadil®
3. How to take Oxyptadil®
4. Possible side effects
5. How to store Oxyptadil®
6. Contents of the pack and other information
1. What Oxyptadil® is and what it is used for
This medicine contains Aviptadil. Aviptadil is a synthetic form of human Vasoactive Intestinal Polypeptide (VIP). VIP is highly concentrated in the lung and reduces lung inflammation. It is used to treat Acute Respiratory Distress Syndrome (ARDS). ARDS is a life-threatening lung injury that allows fluid to leak into the lungs. Breathing becomes difficult and oxygen cannot get into the body.
VIP binds specifically to alveolar type II cells (ATII) in lung alveolus, where it elicits anti-inflammatory/anti-cytokine activity in respiratory distress, acute lung injury, and inflammation. Aviptadil stimulates ATII cells to make more surfactant that must coat the lining of the lungs in order for them to exchange oxygen with the blood; loss of surfactant causes respiratory failure and alveolar collapse.
ARDS results from lung injury. The exact nature of the injury is not always clear. Common injuries are:
- Sepsis, a life-threatening condition occurs when your immune system must work aggressively to fight off infection or trauma
- Pneumonia (viral, bacterial, covid-19, Tuberculosis etc.)
- Inhaling harmful substances
- Trauma to the head, chest or other areas of the body
- Blood transfusions, injury to Pancreas, Near drowning
In the early stages of ARDS, fluid from the smallest blood vessels in the lungs starts to leak into the alveoli—the tiny air sacs where oxygen exchange takes place. The lungs become smaller and stiffer and it becomes hard to breath. The amount of oxygen in the blood falls. This is called hypoxemia. The body becomes starved for oxygen. This harms the brain and other tissues and leads to organ failure.
Patients with ARDS are short of breath, often to a distressing level. They are breathing faster and their heart is beating faster. They may have pain as they try to take a breath. As the oxygen in the blood falls, their fingernails and lips may have a bluish colour.
2. What you need to know before you take Oxyptadil®
Do not take Oxyptadil® if you are allergic to hypersensitive to Aviptadil.
Warnings and precautions
Consult with your doctor before taking Oxyptadil® if you have or have had cardiovascular or cerebrovascular conditions.
If you develop diarrhoea or loose stools during or after treatment, tell your doctor. Do not take any medicine to treat your diarrhoea without first checking with your doctor. If your diarrhoea continues, please inform your doctor.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Driving and using machines
Most people who get ARDS are already at the hospital for infection or trauma.
3. How to administer Oxyptadil®
Your doctor will administer this medicine by an infusion pump in escalating doses for 3 successive days. One vial on Day 1, two vials on Day 2, and three vials on Day 3. The duration of the infusion depends on your body weight. Usually, infusion is administered in 12 hours.
4. Possible side effects
Mild transient flushing of the face or trunk occurs commonly with Aviptadil infusion. Diarrhoea, hypotension, cutaneous flushing, facial flushing and infusion-related reactions may occur with Aviptadil treatment. Some side effects can be serious and need immediate medical attention.
Stop the Aviptadil on worsening the symptoms. If you develop these symptoms and your doctor give medical attention immediately.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Oxyptadil®
Do not take or give this medicine after the expiry date which is stated on the bottle. The expiry date refers to the last day of that month.
This medicine requires to maintain at 2-80C storage conditions. In case any foreign particulate is seen inside the vial do not use the vial for administration.
6. Contents of the pack and other information
What Oxyptadil® contains
The active substance is Avipatdil (150 mcg in 10 mL).
What Oxyptadil® looks like
Oxyptadil® is an injection for Intravenous infusion and comes in a 10 mL vial size.
A vial of 10 ml.