Ozolid SR 1200 Tablets
Therapy Area
Anti Infective
Composition
Each film coated sustained release tablet contains :
Linezolid IP 1200 mg
Excipients q.s.
Colours : Ferric Oxide USP-NF (Yellow) & Titanium Dioxide IP
Description
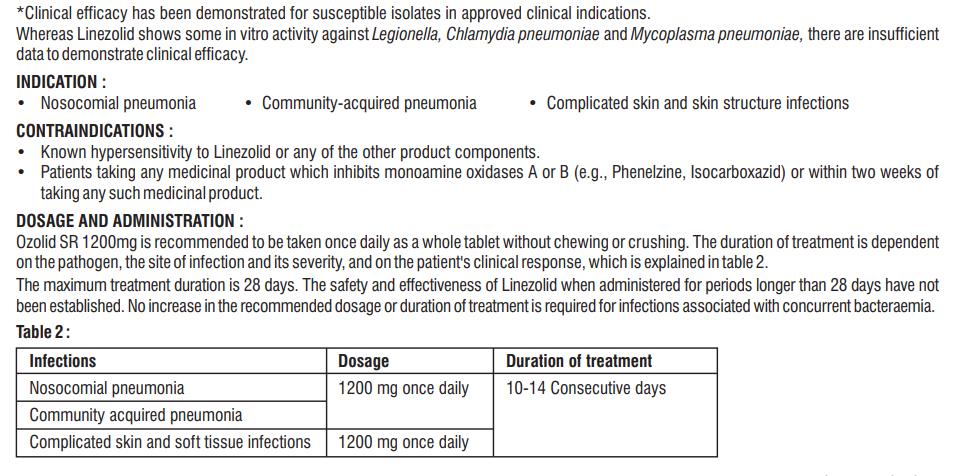
Linezolid, which is a synthetic antibacterial agent of the oxazolidinone class.
Molecular formula : C16H20 FN3O4
Molecular weight : 337.35 g/mol
IUPAC Name : (S)-N-({3-[3-fluoro-4-(morpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)acetamide.
Clinical Pharmacology
Pharmacodynamics
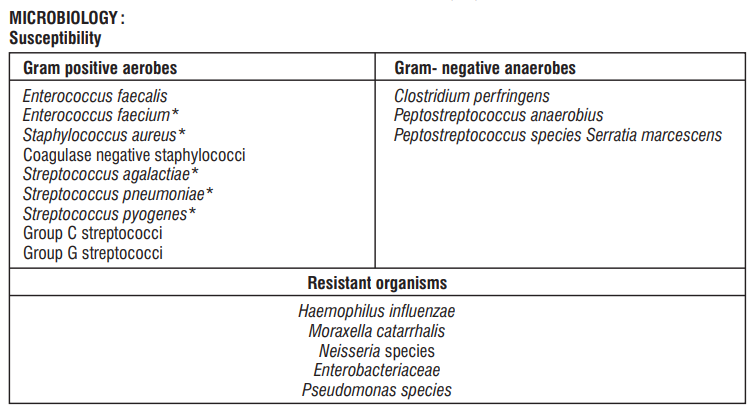
Linezolid is a synthetic, antibacterial agent that belongs to a new class of antimicrobials, the oxazolidinones. It has in vitro activity against aerobic Gram positive bacteria and anaerobic micro-organisms. Linezolid selectively inhibits bacterial protein synthesis via a unique mechanism of action. Specifically, it binds to a site on the bacterial ribosome (23S of the 50S subunit) and prevents the formation of a functional 70S initiation complex which is an essential component of the translation process.
Pharmacokinetics
Linezolid is extensively absorbed after oral dosing. Maximum plasma concentrations are reached approximately 1 to 2 hours after dosing, and the absolute bioavailability is approximately 100%. Absorption is not significantly affected by food. Linezolid is primarily metabolised by oxidation of the morpholine ring resulting mainly in the formation of two inactive open-ring carboxylic acid derivatives; the aminoethoxyacetic acid metabolite and the hydroxyethyl glycine metabolite. In patients with normal renal function or mild to moderate renal insufficiency, Linezolid is primarily excreted under steady-state conditions in the urine.
Bioequivalence Testing :
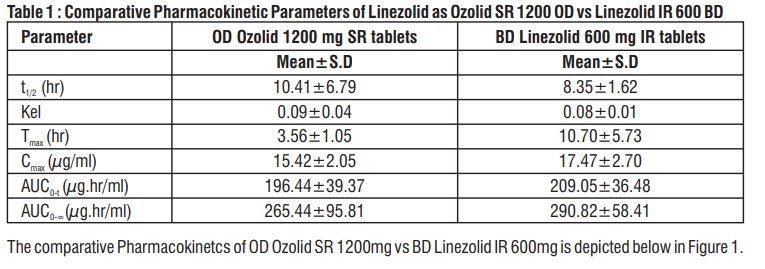
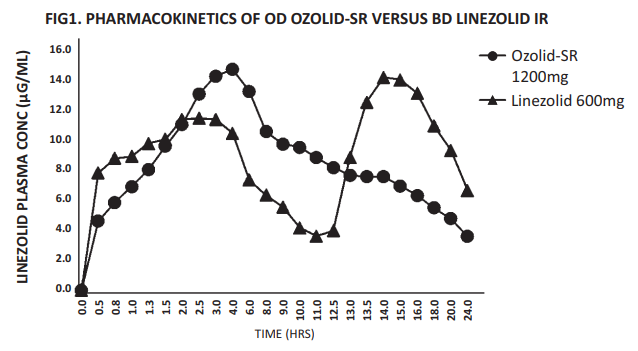
A crossover, comparative bioavailability study of Ozolid SR 1200 given as a single dose (Linezolid 1200 mg Sustained Release Tablet from Zuventus Healthcare Limited) was compared with Linezolid Immediate Release 600 mg x 2 tablet [1 tablet each at 12 hourly and each tablet containing Linezolid 600 mg] to determine the systemic exposure of Linezolid in plasma over a period of 24 hours. It was observed that the Area under the curve of the two formulations was similar proving their bioequivalence as shown in table 1.
Paediatric population
The safety and efficacy of Linezolid in children aged (< 18 years old) has not been established.
Adverse Effects
Most common adverse reactions (>5% of adult and/or pediatric patients treated with Linezolid) include : diarrhea, vomiting, headache, nausea, and anemia
The following undesirable effects have been observed and reported during treatment with Linezolid with the following frequencies : Infections and infestations : Common- candidiasis, oral candidiasis, vaginal candidiasis, fungal infections; Uncommon- vaginitis; Rareantibiotic-associated colitis, including pseudomembranous colitis. Blood and the lymphatic system disorders : Common- anaemia; Uncommon- leucopenia, neutropenia, thrombocytopenia, eosinophilia; Rare-pancytopenia; Frequency not known- myelosuppression, sideroblastic anaemia. Immune system disorders : Frequency not known- anaphylaxis. Metabolism and nutrition disorders : Uncommonhyponatraemia; Frequency not known- lactic acidosis. Psychiatric disorders : Common- insomnia. Nervous system disorders : Commonheadache, taste perversion (metallic taste), dizziness; Uncommon- convulsions, hypoaesthesia, paraesthesia; Frequency not known- serotonin syndrome, peripheral neuropathy. Eye disorders : Uncommon- blurred vision; Rare- changes in visual field defect; Frequency not known- optic neuropathy, optic neuritis, loss of vision, changes in visual acuity, changes in colour vision. Ear and labyrinth disorders : Uncommon- tinnitus. Cardiac disorders : Uncommon- arrhythmia (tachycardia). Vascular disorders : Common-hypertension; Uncommon-transient ischaemic attacks, phlebitis, thrombophlebitis. Gastrointestinal disorders : Common- diarrhoea, nausea, vomiting, localised or general abdominal pain, constipation, dyspepsia; Uncommon- pancreatitis, gastritis, abdominal distention, dry mouth, glossitis, loose stools, stomatitis, tongue discolouration or disorder; Rare- superficial tooth discolouration. Hepato-biliary disorders : Common- abnormal liver function test; increased AST, ALT or alkaline phosphatase; Uncommon- increased total bilirubin. Skin and subcutaneous tissue disorders : Common- pruritus, rash; Uncommon- urticaria, dermatitis, diaphoresis; Frequency not known- bullous disorders such as those described as Stevens-Johnson syndrome and toxic epidermal necrolysis, angioedema, alopecia. Renal and urinary disorders : Common- increased BUN; Uncommon- renal failure, increased creatinine, polyuria. Reproductive system and breast disorders : Uncommon- vulvovaginal disorder. General disorders and administration site conditions : Common- fever, localised pain; Uncommon-chills, fatigue, injection site pain, increased thirst. Investigations : Common- Chemistry- Increased LDH, creatine kinase, lipase, amylase or non fasting glucose. Decreased total protein, albumin, sodium or calcium. Increased or decreased potassium or bicarbonate; Haematology- Increased neutrophils or eosinophils. Decreased haemoglobin, haematocrit or red blood cell count. Increased or decreased platelet or white blood cell counts. Uncommon- Chemistry-Increased sodium or calcium. Decreased non fasting glucose. Increased or decreased chloride. Haematology- Increased reticulocyte count. Decreased neutrophils.
Use in Specific Populations
Pregnancy
Pregnancy Category C : Linezolid should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether Linezolid is excreted in human milk.
Caution should be exercised when Ozolidisadm in istered to an ursing woman.
Usage in pediatrics : Pediatric patients exhibit wider variability in Linezolid clearance and systemic exposure (AUC) compared with adults. In pediatric patients with a sub-optimal clinical response, particularly those with pathogens with MIC of 4 mcg/mL, lower systemic exposure, site and severity of infection, and the underlying medical condition should be considered when assessing clinical response.
Usage in geriatrics : No overall differences in safety or effectiveness were obser ved between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Severe renal impairment (i.e. CLCR < 30 ml/min) : No dose adjustment is required. Linezolid should be used with special caution in patients with severe renal insufficiency who are undergoing dialysis and only when the anticipated benefit is considered to outweigh the theoretical risk.
Hepatic impairment : No dose adjustment is required. However, there are limited clinical data and it is recommended that Linezolid should be used in such patients only when the anticipated benefit is considered to outweigh the theoretical risk.
Drug Interactions
Monoamine Oxidase Inhibitors
Linezolid is a reversible, nonselective inhibitor of monoamine oxidase.
Adrenergic and Serotonergic Agents
Linezolid has the potential for interaction with adrenergic and serotonergic agents.
WARNINGS AND PRECAUTIONS :
Myelosuppression
Myelosuppression (including anemia, leukopenia, pancytopenia, and thrombocytopenia) has been reported in patients receiving Linezolid. Discontinuation of therapy with Linezolid should be considered in patients who develop or have worsening myelosuppression.
Peripheral and Optic Neuropathy
Peripheral and optic neuropathies have been reported in patients treated with Linezolid, primarily in those patients treated for longer than the maximum recommended duration of 28 days. If patients experience symptoms of visual impairment, such as changes in visual acuity, changes in color vision, blurred vision, or visual field defect, prompt ophthalmic evaluation is recommended. Visual function should be monitored in all patients taking Linezolid for extended periods (≥ 3 months) and in all patients reporting new visual symptoms regardless of length of therapy with Linezolid. If peripheral or optic neuropathy occurs, the continued use of Linezolid in these patients should be weighed against the potential risks.
Serotonin Syndrome
Patients taking serotonergic antidepressants should receive Linezolid only if no other therapies
are available. Discontinue serotonergic antidepressants and monitor patients for signs and symptoms of both serotonin syndrome and antidepressant discontinuation.
Mortality Imbalance in an Investigational Study in
Patients with Catheter-Related Bloodstream
Linezolid is not approved and should not be used for the treatment of
patients with catheter-related bloodstream infections or catheter-site infections.
Clostridium difficile Associated Diarrhea
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Linezolid, and may range in severity from mild diarrhea to fatal colitis.
- Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued.
- Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Potential Interactions Producing Elevation of Blood Pressure
Unless patients are monitored for potential increases in blood pressure, Linezolid should not be administered to patients with uncontrolled hypertension, pheochromocytoma, thyrotoxicosis and/or patients taking any of the following types of medications: directly and indirectly acting sympathomimetic agents (e.g., pseudoephedrine), vasopressive agents (e.g., epinephrine, norepinephrine), dopaminergic agents (e.g., dopamine, dobutamine).
Lactic Acidosis
Lactic acidosis has been reported with the use of Linezolid. In reported cases, patients experienced repeated episodes of nausea and vomiting. Patients who develop recurrent nausea or vomiting, unexplained acidosis, or a low bicarbonate level while receiving Linezolid should receive immediate medical evaluation.
Convulsions
ConvulsionshavebeenreportedinpatientswhentreatedwithLinezolid.
Insomeofthesecases, ahistoryof seizuresorrisk factors for seizureswas reported.
Hypoglycemia
Postmarketing cases of symptomatic hypoglycemia have been reported in patients with diabetes mellitus receiving insulin or oral hypoglycemic agents when treated with Linezolid, a reversible, nonselective MAO inhibitor. If hypoglycemia occurs, a decrease in the dose of insulin or oral hypoglycemic agent, or discontinuation of oral hypoglycemic agent, insulin, or Linezolid may be required.
Development of Drug-Resistant Bacteria
Prescribing Linezolid in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Overdosage
In the event of overdosage, supportive care is advised, with maintenance of glomerular filtration. To remove any unabsorbed Linezolid gastric lavage, administration of activated charcoal are recommended. Hemodialysis may facilitate more rapid elimination of Linezolid. In a Phase 1 clinical trial, approximately 30% of a dose of Linezolid was removed during a 3-hour hemodialysis session beginning 3 hours after the dose of Linezolid was administered. Data are not available for removal of Linezolid with peritoneal dialysis or hemoperfusion. Clinical signs of acute toxicity in animals were decreased activity and ataxia in rats and vomiting and tremors in dogs treated with 3000 mg/kg/day and 2000 mg/kg/day, respectively
Storage
Store in a dry place at a temperature not exceeding 30°C.
Protect from light.
Keep out of reach of children.
Shelf-life
Refer on the pack.
Presentation
Alu-Alu blister strip of 5 tablets.
The name of your medicine is OZOLID SR 1200 Tablets. We refer to them as OZOLID Tablets or OZOLID throughout this leaflet.
- Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What OZOLID is and what it is used for
2. What you need to know before you take OZOLID
3. How to take OZOLID
4. Possible side effects
5. How to store OZOLID
6. Contents of the pack and other information
1. What OZOLID is and what it is used for
OZOLID is an antibiotic of the oxazolidinones group that works by stopping the growth of certain bacteria (germs) that cause infections. It is used to treat pneumonia and some infections in the skin or under the skin. It is also used to treat infection of bone in adults.
Your doctor will have decided if OZOLID is suitable to treat your infection.
What you need to know before you take OZOLID
2. Do not take OZOLID:
- If you are allergic to linezolid or any of the other ingredients of this medicine.
- If you are taking or have taken within the last 2 weeks any medicines known as monoamine oxidase inhibitors (MAOIs: for example phenelzine, isocarboxazid, selegiline, moclobemide). These medications may be used to treat depression or Parkinson’s disease.
- If you are breast-feeding. This is because OZOLID passes into breast milk and could affect the baby.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before taking OZOLID.
OZOLID may not be suitable for you if you answer yes to any of the following questions. In this case tell your doctor as he/she will need to check your general health and your blood pressure before and during your treatment or may decide that another treatment is better for you.
Ask your doctor if you are not sure whether these categories apply to you.
- Do you have high blood pressure, whether or not you are taking medicines for this?
- Have you been diagnosed with an overactive thyroid?
- Do you have a tumour of the adrenal glands (phaeochromocytoma) or carcinoid syndrome (caused by tumours of the hormone system with symptoms of diarrhoea, flushing of the skin, wheezing)?
- Do you suffer from manic depression, schizoaffective disorder, mental confusion or other mental problems?
Take special care with OZOLID
Tell your doctor before you take this medicine if you:
- Bruise and bleed easily are anaemic (have low red blood cells)
- Are prone to getting infections
- Have a history of seizures
- Have liver problems or kidney problems particularly if you are on dialysis
- Have diarrhoea
Tell your doctor immediately if during treatment you suffer from:
- Problems with your vision such as blurred vision, changes in colour vision, difficulty in seeing detail or if your field of vision becomes restricted.
- Loss of sensitivity in your arms or legs or a sensation of tingling or pricking in your arms or legs.
- You may develop diarrhoea while taking or after taking antibiotics, including OZOLID. If this becomes severe or persistent or you notice that your stool contains blood or mucus, you should stop taking OZOLID immediately and consult your doctor. In this situation, you should not take medicines that stop or slow bowel movement.
- Recurrent nausea or vomiting, abdominal pain or rapid breathing
Other medicines and OZOLID
There is a risk that OZOLID may sometimes interact with certain other medicines to cause side effects such as changes in blood pressure, temperature or heart rate.
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines.
Tell your doctor if you are taking or have taken within the last 2 weeks the following medicines as OZOLID must not be taken if you are already taking these medicines or have taken them recently (see also Section 2 above ‘Do not take OZOLID’).
- Monoamine oxidase inhibitors (MAOIs for example phenelzine, isocarboxazid, selegiline, moclobemide). These may be used to treat depression or Parkinson’s disease Also tell your doctor if you are taking the following medicines. Your doctor may still decide to give you OZOLID, but will need to check your general health and your blood pressure before and during your treatment.
In other cases, your doctor may decide that another treatment is better for you.
- Decongestant cold or flu remedies containing pseudoephedrine or phenylpropanolamine.
- Some medicines used to treat asthma such as salbutamol, terbutaline, fenoterol.
- Certain antidepressants known as tricyclics or SSRIs (selective serotonin reuptake inhibitors).
- There are many of these, including amitriptyline, citalopram, clomipramine, dosulepin, doxepin, fluoxetine, fluvoxamine, imipramine, lofepramine, paroxetine, sertraline.
- Medicines used to treat migraine such as sumatriptan and zolmitriptan.
- Medicines used to treat sudden, severe allergic reactions such as adrenaline (epinephrine).
- Medicines which increase your blood pressure, such as noradrenaline (norepinephrine), dopamine and dobutamine.
- Medicines used to treat moderate to severe pain, such as pethidine.
- Medicines used to treat anxiety disorders, such as buspirone.
- Medicines that stop blood clotting, such as warfarin.
- An antibiotic called rifampicin.
OZOLID with food, drink and alcohol
- You can take OZOLID either before, during or after a meal.
- Avoid eating large amounts of mature cheese, yeast extracts, or soya bean extracts e.g., soy sauce and drinking alcohol, especially draught beers and wine. This is because OZOLID may react with a substance called tyramine which is naturally present in some foods. This interaction may cause an increase in your blood pressure.
Pregnancy, breast-feeding and fertility
The effect of OZOLID in pregnant women is not known. Therefore, it should not be taken in pregnancy unless advised by your doctor. If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You should not breast-feed when taking OZOLID because it passes into breast milk and could affect the baby.
Driving and using machines
OZOLID may make you feel dizzy or experience problems with your vision. If this happens, do not drive or operate any machinery. Remember that if you are unwell your ability to drive or operate machinery may be affected.
3. How to take OZOLID
Adults
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you. Check with your doctor, pharmacist or nurse if you are not sure. The recommended dose is one film-coated tablet (1200 mg linezolid) once daily. Swallow the film-coated tablet whole with some water. If you are on kidney dialysis, you should take OZOLID after your dialysis treatment. A course of treatment usually lasts 10 to 14 days, but can last up to 28 days. The safety and effectiveness of this medicine have not been established for treatment periods longer than 28 days. Your doctor will decide how long you should be treated. While you are taking OZOLID, your doctor should perform regular blood tests to monitor your blood count. Your doctor should monitor your eyesight if you take OZOLID for more than 28 days.
Use in children and adolescents
OZOLID is not normally used to treat children and adolescents (under 18 years old).
If you take more OZOLID than you should
Tell your doctor or pharmacist immediately.
If you forget to take OZOLID
Take the forgotten tablet as soon as you remember. Take the next film-coated tablet 24 hours after this and continue taking your film-coated tablets every 24 hours. Do not take a double dose to make up for a forgotten film-coated tablet.
If you stop taking OZOLID
Unless your doctor instructs you to stop treatment, it is important to continue taking OZOLID. If you stop and your original symptoms come back tell your doctor or pharmacist immediately.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them Tell your doctor, nurse or pharmacist immediately if you notice any of these side effects during your treatment with OZOLID:
The serious side effects (with frequency in brackets) of OZOLID are:
Severe skin disorder (not known), swelling particularly around the face and neck (not known), wheezing and/or difficulty breathing (not known). This may be the sign of an allergic reaction and it may be necessary for you to stop taking OZOLID. Skin reactions such as red sore skin and flaking (dermatitis) (uncommon), rash (common), itching (common).
Problems with your vision such as blurred vision (uncommon), changes in colour vision (not known), difficulty in seeing detail (not known) or if your field of vision becomes restricted (rare).
Severe diarrhoea containing blood and/or mucus (antibiotic associated colitis including pseudomembranous colitis), which in rare circumstances may develop into complications that are life-threatening (rare).
Recurrent nausea or vomiting, abdominal pain or rapid breathing (not known).
Fits or seizures (uncommon) have been reported with OZOLID. You should let your doctor know if you experience agitation, confusion, delirium, rigidity, tremor, incoordination and seizure while also taking antidepressants known as SSRIs (not known).
Unexplained bleeding or bruising, which may be due to changes in the numbers of certain cells in the blood which may affect blood clotting or lead to anaemia (common).
Changes in numbers of certain cells in the blood which may affect your ability to fight infection (common) some signs of infection include: any fever (common), sore throat (uncommon), mouth ulcers (uncommon) and tiredness (uncommon).
Inflammation of the pancreas (uncommon).
Convulsions (uncommon).
Transient ischaemic attacks (temporary disturbance of blood flow to the brain causing short term symptoms such as loss of vision, leg and arm weakness, slurring of speech and loss of consciousness) (uncommon).
"Ringing" in the ears (tinnitus) (uncommon).
Numbness, tingling or blurred vision have been reported by patients who have been given OZOLID for more than 28 days. If you experience difficulties with your vision you should consult your doctor as soon as possible.
Other side effects include:
Common (may affect up to 1 in 10 people):
- Fungal infections especially vaginal or oral “thrush”
- Headache
- Metallic taste in the mouth
- Diarrhoea, nausea or vomiting
- Changes in some blood test results including those measuring your kidney or liver function or blood sugar levels
- Difficulty in sleeping
- Increased blood pressure
- Anaemia (low red blood cell)
- Dizziness
- Localised or general abdominal pain
- Constipation
- Indigestion
- Localised pain
Uncommon (may affect up to 1 in 100 people):
Inflammation of the vagina or genital area in women
- Sensations such as tingling or feeling numb
- Inflammation of the veins (IV only)
- Swollen, sore, or discoloured tongue
- A need to urinate more often
- Chills
- Feeling thirsty
- Increased sweating
- Changes in proteins, salts or enzymes in the blood which measure kidney or liver function
- Hyponatraemia (low blood sodium levels)
- Kidney failure
- Reduction in platelets
- Abdominal bloating
- Injection site pain
- Increase in creatinine
- Stomach pain
- Changes in heart rate (e.g., increase rate)
Rare (may affect up to 1 in 1,000 people):
- Superficial tooth discolouration, removable with professional dental cleaning (manual descaling)
The following side effects have also been reported (Not known: frequency cannot be estimated from the available data):
- Alopecia (hair loss)
- Decrease of the blood cell count
- Weakness and/or sensory changes
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects to medico@zuventus.com and you can report directly via the national pharmacovigilance program of India by calling on 1800 180 3024.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store OZOLID
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the pack or the blister after ‘EXP’. The expiry date refers to the last day of that month.
Store in a dry place at a temperature not exceeding 30oC. Protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What OZOLID contains
The active substance in this medicine is called linezolid. Each film-coated sustained release tablet contains 1200 mg linezolid and excipients.
The Marketing Authorisation Holder
Zuventus Healthcare Ltd.
Manufacturer
Inventia Healthcare Limited,
F1-F1/1, Additional Ambernath M.I.D.C.,
Ambernath (East)- 421 506 District- Thane
© Zuventus Healthcare Ltd., 2020. All rights reserved.