Pansa D Tablets
Therapy Area
Gastrointestinal
1.0 Generic Name
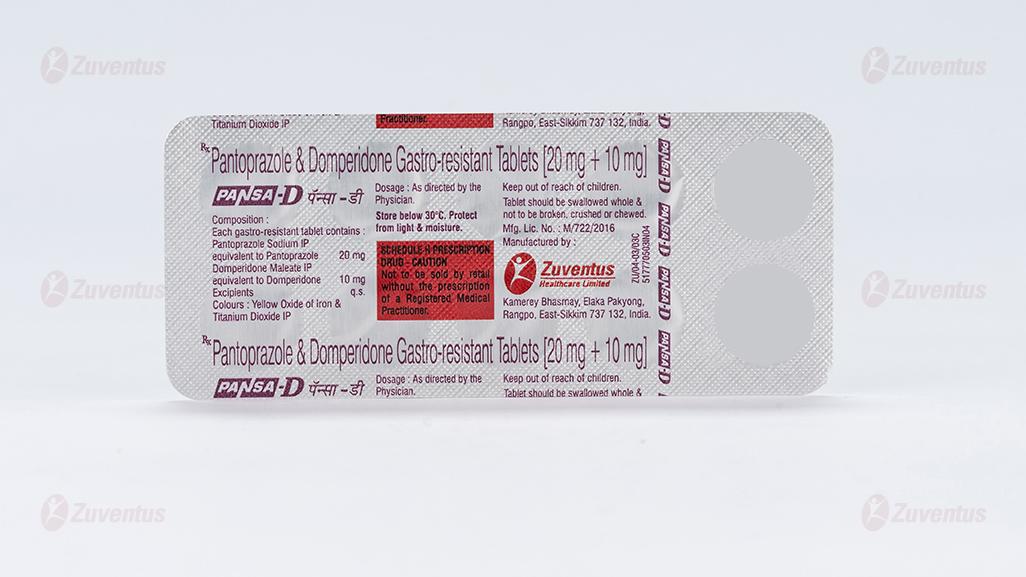
Pantoprazole & Domperidone Gastro-resistant Tablets (20 mg + 10 mg)
2.0 Qualitative and quantitative composition
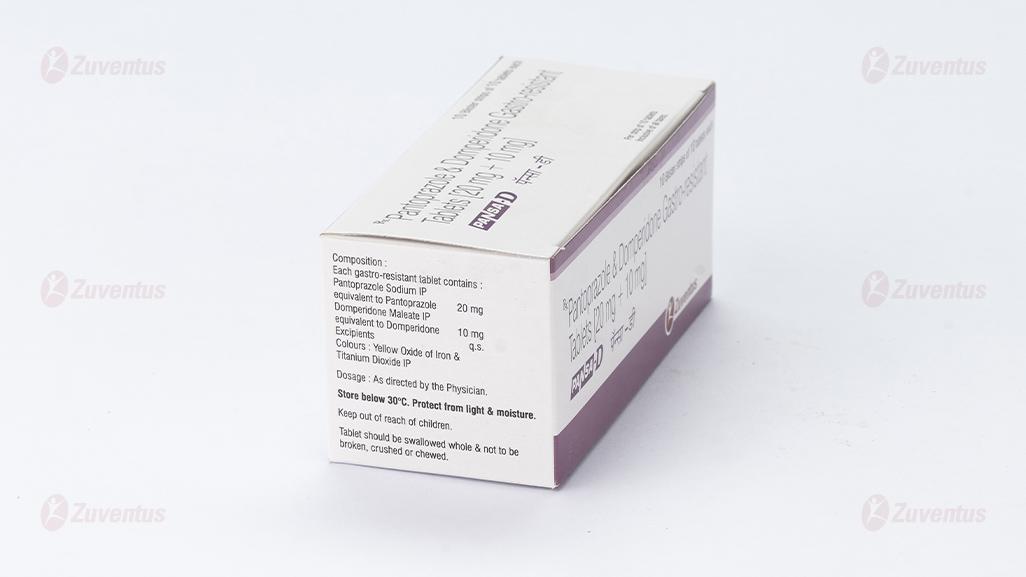
Each gastro-resistant tablet contains :
Pantoprazole Sodium IP
equivalent to Pantoprazole 20 mg
Domperidone Maleate IP
equivalent to Domperidone 10 mg
Excipients q.s.
Colours : Yellow Oxide of Iron & Titanium Dioxide IP
3.0 Dosage form and strength
Tablets
4.0 Clinical particulars
4.1 Therapeutic indication
For the treatment of GERD not responding to pantoprazole.
4.2 Posology and method of administration
One tablet to be taken 1-3 times a day. The tablets should not be chewed or crushed, and should be swallowed whole 1 hour before a meal with some water.
4.3 Contraindications
- known hypersensitivity to the active substance pantoprazole, domperidone or to substituted benzimidazoles or to any excipients listed in formulation.
- in patients with moderate or severe hepatic impairment
- renal impairment
- in patients who have known existing prolongation of cardiac conduction intervals, particularly QTc, patients with significant electrolyte disturbances or underlying cardiac diseases such as congestive heart failure.
- prolactin-releasing pituitary tumour (prolactinoma)
- co-administration with QT-prolonging drugs, at the exception of apomorphine
- co-administration with potent CY3A4 inhibitors (regardless of their QT prolonging effects) when stimulation of gastric motility could be harmful : gastro-intestinal haemorrhage, mechanical obstruction or perforation.
4.4 Special warnings and precautions for use
In patients with severe liver impairment the liver enzymes should be monitored regularly during treatment with pantoprazole, particularly on long-term use. In the case of a rise of the liver enzymes the treatment should be discontinued.
The use of pantoprazole as a preventive of gastroduodenal ulcers induced by non-selective non-steroidal anti-inflammatory drugs (NSAIDs) should be restricted to patients who require continued NSAID treatment and have an increased risk to develop gastrointestinal complications. The increased risk should be assessed according to individual risk factors, e.g. high age (>65 years), history of gastric or duodenal ulcer or upper gastrointestinal bleeding.
Symptomatic response to pantoprazole may mask the symptoms of gastric malignancy and may delay diagnosis. In the presence of any alarm symptom (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis, anaemia or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded. Further investigation is to be considered if symptoms persist despite adequate treatment.
Pantoprazole may reduce the absorption of vitamin B12 due to hypo- or achlorhydria. This should be considered in patients with reduced body stores or risk factors for reduced vitamin B12 absorption on long-term therapy or if respective clinical symptoms are observed.
In long-term treatment with pantoprazole, especially when exceeding 1 year, patients should be kept under regular surveillance.
Treatment with pantoprazole may lead to a slightly increased risk of gastrointestinalinfections caused by bacteria such as Salmonella and Campylobacter or C. difficile.
For patients expected to be on prolonged pantoprazole treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), healthcare professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically thereafter.
PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
PPIs are associated with very infrequent cases of Subacute Cutaneous Lupus Erythematosus (SCLE). If lesions occur, especially in sun exposed areas of the skin, and if accompanied by arthralgia, the patient should seek medical help promptly and the healthcare professional should consider stopping pantoprazole.
Increased CgA level may interfere with investigations for neuroendocrine tumours. To avoid this interference, pantoprazole treatment should be stopped for at least 5 days before CgA measurements. If CgA and gastrin levels have not returned to reference range after initial measurement, measurements should be repeated 14 days after cessation of proton pump inhibitor treatment.
The risk of neurological side effects with domperidone is higher in young children. Overdosing may cause extra-pyramidal symptoms, but other causes should be taken into consideration.
Domperidone may be associated with an increased risk of serious ventricular arrhythmias or sudden cardiac death. Contraindicated in patients with known existing prolongation of cardiac conduction intervals, particularly QTc, in patients with significant electrolyte disturbances (hypokalaemia, hyperkalaemia, hypomagnesaemia), or bradycardia, or in patient with underlying cardiac diseases such as congestive heart failure due to increased risk of ventricular arrhythmia.
4.5 Drugs interactions
Pantoprazole
Medicinal products with pH-dependent absorption pharmacokinetics
Because of profound and long lasting inhibition of gastric acid secretion, pantoprazole may interfere with the absorption of other medicinal products where gastric pH is an important determinant of oral availability, e.g. some azole antifungals such as ketoconazole, itraconazole, posaconazole and other medicinal products such as erlotinib
HIV protease inhibitors
Co-administration of pantoprazole is not recommended with HIV protease inhibitors for which absorption is dependent on acidic intragastric pH such as atazanavir due to significant reduction in their bioavailability.
If the combination of HIV protease inhibitors with a proton pump inhibitor is judged unavoidable, close clinical monitoring (e.g virus load) is recommended. A pantoprazole dose of 20 mg per day should not be exceeded. Dosage of the HIV protease inhibitor may need to be adjusted.
Coumarin anticoagulants (phenprocoumon or warfarin)
Co-administration of pantoprazole with warfarin or phenprocoumon did not affect the pharmacokinetics of warfarin, phenprocoumon or INR. However, there have been reports of increased INR and prothrombin time in patients receiving PPIs and warfarin or phenprocoumon concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding, and even death. Patients treated with pantoprazole and warfarin or phenprocoumon may need to be monitored for increase in INR and prothrombin time.
Methotrexate
Concomitant use of high dose methotrexate (e.g. 300 mg) and proton-pump inhibitors has been reported to increase methotrexate levels in some patients. Therefore in settings where high-dose methotrexate is used, for example cancer and psoriasis, a temporary withdrawal of pantoprazole may need to be considered. Other interactions studies
Pantoprazole is extensively metabolized in the liver via the cytochrome P450 enzyme system. The main metabolic pathway is demethylation by CYP2C19 and other metabolic pathways include oxidation by CYP3A4.
Interaction studies with medicinal products also metabolized with these pathways, like carbamazepine, diazepam, glibenclamide, nifedipine, and an oral contraceptive containing levonorgestrel and ethinyl oestradiol did not reveal clinically significant interactions.
An interaction of pantoprazole with other medicinal products or compounds, which are metabolized using the same enzyme system, cannot be excluded.
Results from a range of interaction studies demonstrate that pantoprazole does not affect the metabolism of active substances metabolised by CYP1A2 (such as caffeine, theophylline), CYP2C9 (such as piroxicam, diclofenac, naproxen), CYP2D6 (such as metoprolol), CYP2E1 (such as ethanol) or does not interfere with p-glycoprotein related absorption of digoxin.
There were no interactions with concomitantly administered antacids.
Interaction studies have also been performed by concomitantly administering pantoprazole with the respective antibiotics (clarithromycin, metronidazole, amoxicillin) No clinically relevant interactions were found.
Medicinal products that inhibit or induce CYP2C19
Inhibitors of CYP2C19 such as fluvoxamine could increase the systemic exposure of pantoprazole. A dose reduction may be considered for patients treated long-term with high doses of pantoprazole, or those with hepatic impairment.
Enzyme inducers affecting CYP2C19 and CYP3A4 such as rifampicin and St John´s wort (Hypericum perforatum) may reduce the plasma concentrations of PPIs that are metabolized through these enzyme systems.
Domperidone
The main metabolic pathway of domperidone is through CYP3A4. In vitro data suggest that the concomitant use of drugs that significantly inhibit this enzyme may result in increased plasma levels of domperidone.
Increased risk of occurrence of QT-interval prolongation, due to pharmacodynamic and/or pharmacokinetic interactions. Concomitant use of the following substances is contraindicated
QTc-prolonging medicinal products
- anti-arrhythmics class IA (e.g., disopyramide, hydroquinidine, quinidine)
- anti-arrhythmics class III (e.g., amiodarone, dofetilide, dronedarone, ibutilide, sotalol)
- certain antipsychotics (e.g., haloperidol, pimozide, sertindole)
- certain antidepressants (e.g., citalopram, escitalopram)
- certain antibiotics (e.g. , erythromycin, levofloxacin, moxifloxacin, spiramycin)
- certain antifungal agents (e.g., pentamidine)
- certain antimalarial agents (in particular halofantrine, lumefantrine)
- certain gastro-intestinal medicines (e.g., cisapride, dolasetron, prucalopride)
- certain antihistaminics (e.g., mequitazine, mizolastine)
- certain medicines used in cancer (e.g., toremifene, vandetanib, vincamine)
- certain other medicines (e.g., bepridil, diphemanil, methadone)
- apomorphine, unless the benefit of the co-administration outweighs the risks, and only if the recommended precautions for co-administration are strictly fulfilled.
Potent CYP3A4 inhibitors (regardless of their QT prolonging effects), i.e :
- protease inhibitors
- systemic azole antifungals
- some macrolides (erythromycin, clarithromycin and telithromycin)
Concomitant use of the following substances is not recommended
Moderate CYP3A4 inhibitors i.e. diltiazem, verapamil and some macrolides.
Concomitant use of the following substances requires caution in use
Caution with bradycardia and hypokalaemia-inducing drugs, as well as with the following macrolides involved in QT-interval prolongation : azithromycin and roxithromycin (clarithromycin is contraindicated as it is a potent CYP3A4 inhibitor).
The above list of substances is representative and not exhaustive.
Separate in vivo pharmacokinetic/pharmacodynamic interaction studies with oral ketoconazole or oral erythromycin in healthy subjects confirmed a marked inhibition of domperidone's CYP3A4 mediated first pass metabolism by these drugs
With the combination of oral domperidone 10mg four times daily and ketoconazole 200mg twice daily, a mean QTc prolongation of 9.8 msec was seen over the observation period, with changes at individual time points ranging from 1.2 to 17.5 msec. With the combination of domperidone 10mg four times daily and oral erythromycin 500mg three times daily, mean QTc over the observation period was prolonged by 9.9 msec, with changes at individual time points ranging from 1.6 to 14.3 msec. Both the Cmax and AUC of domperidone at steady state were increased approximately three-fold in each of these interaction studies. In these studies domperidone monotherapy at 10mg given orally four times daily resulted in increases in mean QTc of 1.6 msec (ketoconazole study) and 2.5 msec (erythromycin study), while Ketoconazole monotherapy (200mg twice daily) led to increases in QTc of 3.8 and 4.9 msec, respectively, over the observation period.
4.6 Use in special populations
Pregnancy
Pantoprazole : There are no adequate and well-controlled studies in pregnant women. Domperidone : There are limited post-marketing data of its use in pregnant women. A study in rats has shown reproductive toxicity at a high, maternally toxic dose. Because animal reproduction studies are not always predictive of human response, Pansa D tablet should be used during pregnancy only if clearly needed.
Lactation
There is insufficient data on the excretion of pantoprazole in human milk but excretion into human milk has been reported. Domperidone is excreted in human milk and breast-fed infants receive less than 0.1% of the maternal weight-adjusted dose. Caution should be exercised in case of QTc prolongation risk factor in breast-fed infants.
A decision should be made whether to discontinue breast-feeding or to discontinue/abstain from Pansa D therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the women.
Pediatric
Pansa D tablets are not recommended in children below 12 years of age.
Hepatic impairment
A daily dose of 20 mg pantoprazole should not be exceeded in patients with severe liver impairment. Domperidone is contraindicated in moderate or severe hepatic impairment. Hence, a Pansa D tablet is contraindicated in patients with moderate or severe hepatic impairment.
Renal impairment
In subjects with renal insufficiency (creatinine clearance<30 ml/min/1.73m2) the elimination half-life of domperidone was increased from 7.4 to 20.8 hours. Since very little unchanged drug (approx. 1%) is excreted via the kidneys, it is unlikely that the dose of a single administration needs to be adjusted in patients with renal insufficiency. However, on repeated administration, the dosing frequency of Pansa D should be reduced to once or twice daily depending on severity of the impairment.
4.7 Effects on ability to drive and use machines
Pansa D tablet has no or negligible influence on the ability to drive and use machines. Adverse drug reactions such as dizziness and visual disturbances may occur. If affected, patients should not drive or operate machines.
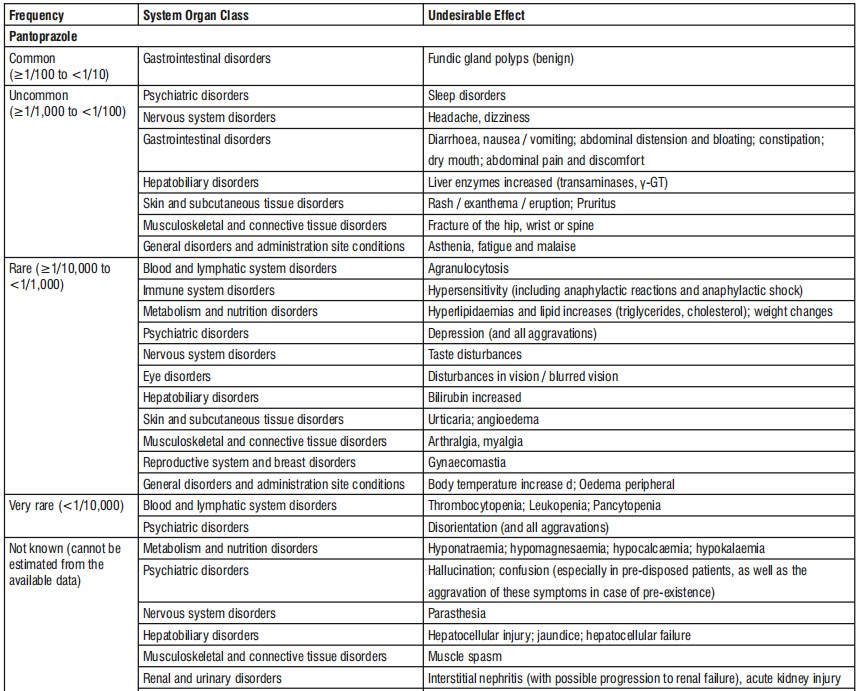
4.8 Undesirable effects

Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Systemic exposure with up to pantoprazole 240 mg administered intravenously over 2 minutes, were well tolerated. As pantoprazole is extensively protein bound, it is not readily dialysable. Symptoms of overdosage with domperidone may include agitation, altered consciousness, convulsions, disorientation, somnolence and extrapyramidal reactions. In the event of overdose, standard symptomatic treatment should be given immediately. Gastric lavage as well as the administration of activated charcoal may be useful. ECG monitoring should be undertaken, because of the possibility of QT interval prolongation. Close medical supervision and supportive therapy is recommended. Anticholinergic, anti-parkinson drugs may be helpful in controlling the extrapyramidal reactions.
5.0 Pharmacological properties
5.1 Mechanism of Action
Pantoprazole is a proton pump inhibitor (PPI) that suppresses the final step in gastric acid production by covalently binding to the (H+,K+)-ATPase enzyme system at the secretory surface of the gastric parietal cell. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. The binding to the (H+,K+)-ATPase results in a duration of antisecretory effect that persists longer than 24 hours. Domperidone binds to dopamine receptor D2 (D2R) expressed by peripheral neurons; this inhibits dopamine binding and D2R-mediated signaling. Inhibition of peripheral D2R signaling prevents or relieves various gastrointestinal (GI) symptoms, such as nausea and vomiting, and may help relief reflux and symptoms of a variety of other upper GI disorders.
5.2 Pharmacodynamicproperties
Pantoprazole
The fasting gastrin values increase under pantoprazole. On short-term use, in most cases they do not exceed the upper limit of normal. During long-term treatment, gastrin levels double in most cases. An excessive increase, however, occurs only in isolated cases. As a result, a mild to moderate increase in the number of specific endocrine (ECL) cells in the stomach is observed in a minority of cases during long-term treatment (simple to adenomatoid hyperplasia). However, according to the studies conducted so far, the formation of carcinoid precursors (atypical hyperplasia) or gastric carcinoids as were found in animal experiments have not been observed in humans. During treatment with antisecretory medicinal products, serum gastrin increases in response to the decreased acid secretion. Also Chromogranin A (CgA) increases due to decreased gastric acidity.
Domperidone
Domperidone does not readily cross the blood brain barrier. In domperidone users, especially in adults, extra pyramidal side effects are very rare, but domperidone promotes the release of prolactin from the pituitary. Studies have shown oral domperidone to increase lower oesophageal pressure, improve antroduodenal motility and accelerate gastric emptying. There is no effect on gastric secretion. When domperidone was administered in healthy subjects at up to 80 mg/day (i.e., more than twice the maximum recommended dosing) in QT study performed in accordance with ICH-E14 guidelines, no clinically relevant QTc effects were observed.
5.3 Pharmacokinetic properties

6.0 Nonclinical properties
No known animal toxicology data
7.0 Description

8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf-life
24 Months.
8.3 Packaging information
A blister strip of 10 tablets.
8.4 Storage and handing instructions
Store protected from light & moisture at a temperature not exceeding 30°C.
Keep out of reach of children.
9.0 Patient Counselling Information
- Ask the patient to report any adverse events.
- Not to exceed the stated recommended daily dose.
12.0 Date of revision
14.05.2021
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, pharmacist or nurse.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What Pansa D is and what it is used for
2. What you need to know before you take Pansa D
3. How to take Pansa D
4. Possible side effects
5. How to store Pansa D
6. Contents of the pack and other information
1. What Pansa D is and what it is used for
Pansa D is the combination of Pantoprazole & Domperidone. Pantoprazole is a selective “proton pump inhibitor”, a medicine which reduces the amount of acid produced in your stomach. It is used for treating acid-related diseases of the stomach and intestine. Domperidone as the active ingredient, which belongs to a group of medicines called 'dopamine antagonists'.
Pansa D is used for the treatment of GERD not responding to pantoprazole.
2. What you need to know before you take Pansa D
Do not take Pansa D if you are
If you are allergic to pantoprazole, domperidone or to any of the other ingredients listed in the formulation
If you are allergic to medicines containing other proton pump inhibitors.
You have black, tarry bowel motions (stools) or notice blood in your bowel motions.
This could be a sign of bleeding in the stomach or intestines You have a blockage or tear in your intestines You have a tumour of the pituitary gland called a prolactinoma. have a disorder known as phenylketonuria (a metabolic disorder) orodispersible tablets should not be used as they contain aspartamine
if you have a moderate or severe liver disease
if your ECG (electrocardiogram) shows a heart problem called "prolonged QT corrected interval"
if you have or had a problem where your heart cannot pump the blood round your body as well as it should (condition called heart failure).
if you have a problem that gives you a low level of potassium or magnesium, or a high level of potassium in your blood.
Do not take Pansa D tablets if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist before taking this medicine. If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before taking Pansa D tablet. Do this even if they have applied in the past.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before taking Pansa D
- If you have severe liver problems. Please tell your doctor if you ever had problems with your liver in the past. He will check your liver enzymes more frequently, especially when you are taking Pantoprazole as a long-term treatment. In the case of a rise of liver enzymes the treatment should be stopped.
- If you have reduced body stores or risk factors for reduced vitamin B12 and receive long-term treatment with pantoprazole. As with all acid reducing agents, pantoprazole may lead to a reduced absorption of vitamin B12.
- If you are taking HIV protease inhibitors such as atazanavir (for the treatment of HIV-infection) at the same time as pantoprazole, ask your doctor for specific advice.
- Taking a proton pump inhibitor like pantoprazole, especially over a period of more than one year, may slightly increase your risk of fracture in the hip, wrist or spine. Tell your doctor if you have osteoporosis or if you are taking corticosteroids (which can increase the risk of osteoporosis).
- If you are on Pantoprazole for more than three months it is possible that the levels of magnesium in your blood may fall. Low levels of magnesium can be seen as fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness, increased heart rate. If you get any of these symptoms, please tell your doctor promptly. Low levels of magnesium can also lead to a reduction in potassium or calcium levels in the blood. Your doctor may decide to perform regular blood tests to monitor your levels of magnesium.
- If you have ever had a skin reaction after treatment with a medicine similar to Pantoprazole that reduces stomach acid.
- If you get a rash on your skin, especially in areas exposed to the sun tell your doctor as soon as you can, as you may need to stop your treatment with Pantoprazole. Remember to also mention any other ill-effects like pain in your joints.
- if you are due to have a specific blood test (Chromogranin A).
- if you suffer from liver problems (liver function impairment or failure)
- if you suffer from kidney problems (kidney function impairment or failure).
It is advisable to ask your doctor for advice in case of prolonged treatment as you may need to take a lower dose or take this medicine less often, and your doctor may want to examine you regularly. Domperidone may be associated with an increased risk of heart rhythm disorder and cardiac arrest. This risk may be more likely in those over 60 years old or taking doses higher than 30mg per day. The risk also increases when domperidone is given together with some drugs. Tell your doctor or pharmacist if you are taking drugs to treat infection (fungal infections or bacterial infection) and/or if you have heart problems or AIDS/HIV. Domperidone should be used at the lowest effective dose. While taking domperidone, contact your doctor if you experience heart rhythm disorders such as palpitations, trouble breathing, loss of consciousness. Treatment with domperidone should be stopped.
Adolescents weighing less than 35 kg and children
Pansa D should not be given to adolescents 12 years of age and older weighing less than 35 kg, or in any children less than 12 years of age, as it is not effective in these age groups.
Tell your doctor immediately, before or after taking this medicine, if you notice any of the following
An unintentional loss of weight
Vomiting, particularly if repeated
Vomiting blood; this may appear as dark coffee grounds in your vomit
You notice blood in your stools; which may be black or tarry in appearance Difficulty in swallowing
or pain when swallowing You look pale and feel weak (anaemia) Chest pain,
Stomach pain, severe and/or persistent diarrhoea, because
this medicine has been associated with a small increase in infectious diarrhoea.
Your doctor may decide that you need some tests to rule out malignant disease because pantoprazole also alleviates the symptoms of cancer and could cause delay in diagnosing it. If your symptoms continue in spite of your treatment, further investigations will be considered. If you take Pantoprazole on a long-term basis (longer than 1 year) your doctor will probably keep you under regular surveillance. You should report any new and exceptional symptoms and circumstances whenever you see your doctor.
Other medicines and Pansa D
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription. This is because Pantoprazole may influence the effectiveness of other medicines, so tell your doctor if you are taking:
Medicines such as ketoconazole, itraconazole and posaconazole (used to treat fungal infections) or erlotinib (used for certain types of cancer) because Pantoprazole may stop these and other medicines from working properly.
Warfarin and phenprocoumon, which affect the thickening, or thinning of the blood. You may need further checks.
Medicines used to treat HIV-infection, such as atazanavir.
Methotrexate (used to treat rheumatoid arthritis, psoriasis, and cancer) – if you are taking methotrexate your doctor may temporarily stop your Pantoprazole treatment because pantoprazole can increase levels of methotrexate in the blood.
Fluvoxamine (used to treat depression and other psychiatric diseases) – if you are taking fluvoxamine your doctor may reduce the dose.
Rifampicin (used to treat infections).
St John’s wort (Hypericum perforatum) (used to treat mild depression).
Do not take Domperidone tablet if you are taking medicine to treat:
fungal infections such as azole antifungals, specifically oral ketoconazole, fluconazole or voriconazole
bacterial infections, specifically erythromycin, clarithromycin, telithromycin,moxifloxacin, pentamidine (these are antibiotics)
heart problems or high blood pressure (e.g., amiodarone, dronedarone, quinidine, disopyramide, dofetilide, sotalol, diltiazem, verapamil)
psychoses (e.g., haloperidol, pimozide, sertindole)
depression (e.g., citalopram, escitalopram)
gastro-intestinal disorders (e.g., cisapride, dolasetron, prucalopride)
allergy (e.g., mequitazine, mizolastine)
malaria (in particular halofantrine)
AIDS/HIV (protease inhibitors)
Hepatitis C (e.g., telaprevir)
cancer (e.g., toremifene, vandetanib, vincamine)
certain other medicines (e.g., bepridil, diphemanil, methadone)
Before you use Domperidone tablet and apomorphine, your doctor will ensure that you tolerate both medicines when used simultaneously. Ask your doctor or specialist for a personalised advice. Tell your doctor or pharmacist if you are taking drugs to treat infection, heart problems or AIDS/HIV. It is important to ask your doctor or pharmacist if Domperidone tablet is safe for you when you are taking any other medicines, including medicines obtained without prescription.
Pregnancy
Pantoprazole: There are no adequate and well-controlled studies in pregnant women.
Domperidone: There are limited post-marketing data of its use in pregnant women. A study in rats has shown reproductive toxicity at a high, maternally toxic dose.
Because animal reproduction studies are not always predictive of human response, Pansa D tablet should be used during pregnancy only if clearly needed.
Lactation
There is insufficient data on the excretion of pantoprazole in human milk but excretion into human milk has been reported.
Domperidone is excreted in human milk and breast-fed infants receive less than 0.1% of the maternal weight-adjusted dose. Caution should be exercised in case of QTc prolongation risk factor in breast-fed infants.
A decision should be made whether to discontinue breast-feeding or to discontinue/abstain from Pansa D therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the women.
Driving and using machines
Pansa D has no or negligible influence on the ability to drive and use machines. If you experience side effects like dizziness or disturbed vision, you should not drive or operate machines.
3. How to take Pansa D
One tablet to be taken 1-3 times a day. The tablets should not be chewed or crushed, and should be swallowed whole 1 hour before a meal with some water.
Pediatric
Pansa D tablets are not recommended in children below 12 years of age.
Hepatic impairment
A daily dose of 20 mg pantoprazole should not be exceeded in patients with severe liver impairment.
Domperidone is contraindicated in moderate or severe hepatic impairment.
Hence, a Pansa D tablet is contraindicated in patients with moderate or severe hepatic impairment.
Renal impairment
In subjects with renal insufficiency (creatinine clearance<30 ml/min/1.73m2) the elimination half-life of domperidone was increased from 7.4 to 20.8 hours. Since very little unchanged drug (approx. 1%) is excreted via the kidneys, it is unlikely that the dose of a single administration needs to be adjusted in patients with renal insufficiency. However, on repeated administration, the dosing frequency of Pansa D should be reduced to once or twice daily depending on severity of the impairment.
If you take more Pansa D than you should
Consult your doctor or pharmacist. There are no known symptoms of overdose.
If you forget to take Pansa D
Do not take a double dose to make up for a forgotten dose. Take your next, normal dose at the usual time.
If you stop taking Pansa D
Do not stop taking these tablets without first talking to your doctor or pharmacist.
4. Possible Side Effects
Pantoprazole
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you get any of the following side effects, stop taking these tablets and tell your doctor immediately, or contact the casualty department at your nearest hospital:
Serious allergic reactions (frequency rare: may affect up to 1 in 1,000 people): swelling of the tongue and/or throat, difficulty in swallowing, hives (nettle rash), difficulties in breathing, allergic facial swelling (Quincke’s oedema / angioedema), severe dizziness with very fast heartbeat and heavy sweating.
Serious skin conditions (frequency not known: frequency cannot be estimated from the available data): blistering of the skin and rapid deterioration of your general condition, erosion (including slight bleeding) of eyes, nose, mouth/lips or genitals (Stevens-Johnson-Syndrome, Lyell-Syndrome, Erythema multiforme), and sensitivity to light.
Other serious conditions (frequency not known: frequency cannot be estimated from the available data): yellowing of the skin or whites of the eyes (severe damage to liver cells, jaundice) or fever, rash, and enlarged kidneys sometimes with painful urination, and lower back pain (serious inflammation of the kidneys), possibly leading to kidney failure.
Other side effects are:
Common (may affect up to 1 in 10 people) Benign polyps in the stomach.
Uncommon (may affect up to 1 in 100 people) Headache; dizziness; diarrhoea; feeling sick, vomiting; bloating and flatulence (wind); constipation; dry mouth; abdominal pain and discomfort; skin rash, exanthema, eruption; itching; feeling weak, exhausted or generally unwell; sleep disorders; fracture in the hip, wrist or spine.
Rare (may affect up to 1 in 1,000 people) Distortion or complete lack of the sense of taste; disturbances in vision such as blurred vision; hives; pain in the joints; muscle pains; weight changes; raised body temperature; high fever; swelling of the extremities (peripheral oedema); allergic reactions; depression; breast enlargement in males.
Very Rare (may affect up to 1 in 10,000 people) Disorientation.
Not known (frequency cannot be estimated from the available data) Hallucination, confusion (especially in patients with a history of these symptoms); decreased sodium level in blood, decreased magnesium level in blood, feeling of tingling, prickling, pins and needles, burning sensation or numbness, rash, possibly with pain in the joints, inflammation in the large bowel, that causes persistent watery diarrhoea.
Side effects identified through blood tests:
Uncommon (may affect up to 1 in 100 people) an increase in liver enzymes.
Rare (may affect up to 1 in 1,000 people) an increase in bilirubin; increased fat levels in blood; sharp drop in circulating granular white blood cells, associated with high fever.
Very Rare (may affect up to 1 in 10,000 people) a reduction in the number of blood platelets, which may cause you to bleed or bruise more than normal; a reduction in the number of white blood cells, which may lead to more frequent infections; coexisting abnormal reduction in the number of red and white blood cells, as well as platelets.
Domperidone
Uncommon (may affect up to 1 in 100 people): Involuntary movements of the face or arms and legs, excessive trembling, excessive muscle stiffness or muscle spasm
Not known (frequency cannot be estimated from the available data):
Seizures
a type of reaction that may occur soon after administration and is recognised by skin rash, itching, shortness of breath, and/or a swollen face.
a severe hypersensitivity reaction that may occur soon after administration that is characterised by hives, itching, flushing, fainting, and difficulty breathing among other possible symptoms.
disorders of the cardiovascular system: heart rhythm disorders (rapid or irregular heart beat) have been reported; if this happens, you should stop the treatment immediately.
Domperidone may be associated with an increased risk of heart rhythm disorder and cardiac arrest. This risk may be more likely in those over 60 years old or taking doses higher than 30 mg per day. Domperidone should be used at the lowest effective dose. Stop treatment with Domperidone and contact your doctor immediately if you experience any of the unwanted events described above.
Other unwanted effects that have been observed with Domperidone are listed below:
Common (may affect up to 1 in 10 people): Dry mouth
Uncommon (may affect up to 1 in 100 people): anxiety, agitation, nervousness, loss of interest in sex or diminished interest in sex, headache, sleepiness, diarrhoea, rash, itchiness, hives, Painful or tender breasts, milk discharge from breasts, a general feeling of weakness, feeling dizzy
Not known (frequency cannot be estimated from the available data): Upward movement of the eyes, stopped menstrual periods in women, enlarged breasts in men, inability to urinate, changes in certain laboratory test results, restless legs syndrome (uncomfortable feeling, with an irresistible urge to move your legs, and sometimes arms and other parts of your body).
Some patients who have used Domperidone for conditions and dosages requiring medical oversight have experienced the following unwanted effects: Restlessness; swollen or enlarged breasts, unusual discharge from breasts, irregular menstrual periods in women, difficulty breastfeeding, depression, hypersensitivity.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Pansa D
Store protected from light & moisture at a temperature not exceeding 30°C.
Keep out of reach of children.
Do not use the tablets after the expiry date is stated on the label. Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
Each gastro-resistant tablet contains -
Pantoprazole Sodium IP equivalent to Pantoprazole 20 mg
Domperidone Maleate IP equivalent to Domperidone 10 mg
Excipients q.s.
Colours : Yellow Oxide of Iron and Titanium Dioxide IP