Pansa DSR Capsules
Therapy Area
Gastrointestinal
1.0 Generic name
Pantoprazole Gastro-resistant and Domperidone Prolonged-release Capsules IP [40 mg + 30 mg]
2.0 Qualitative and quantitative composition
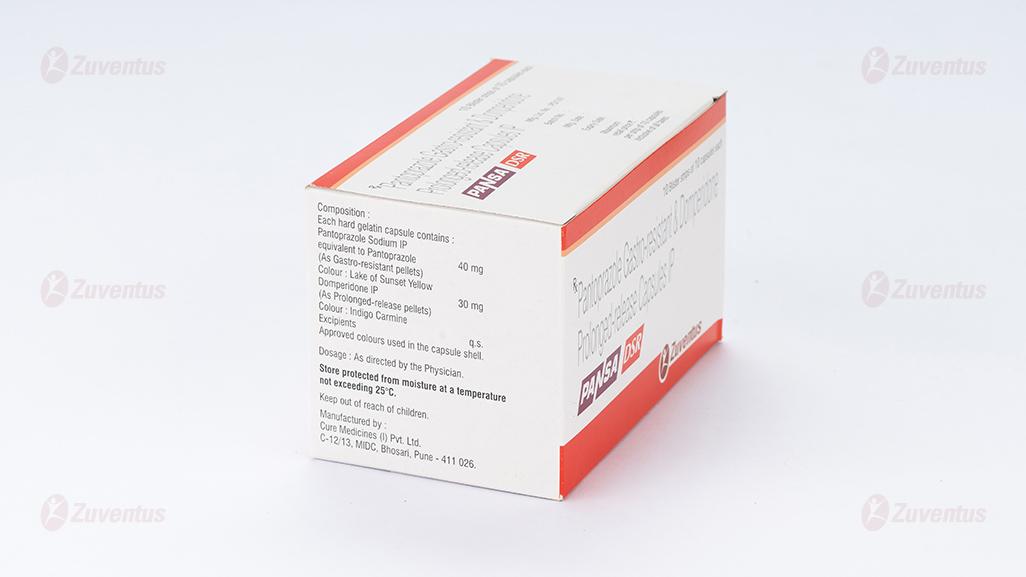
Each hard gelatin capsule contains :
Pantoprazole Sodium IP
equivalent to Pantoprazole 40 mg
(As Gastro-resistant pellets)
Colour : Indigo Carmine
Domperidone IP 30 mg
(As Prolonged-release pellets)
Colour : Lake of Sunset Yellow
Approved colours used in the capsule shell.
3.0 Pharmaceutical form and strength
Capsule, Pantoprazole (40 mg) and Domperidone (30 mg).
4.0 Clinical particulars
4.1 Therapeutic indications
For the treatment gastro-esophageal reflux disease (GERD) not responding adequately to Pantoprazole alone.
4.2 Posology and method of administration
1 Capsule to be administered once daily.
Pansa DSR capsules should be administered on empty stomach, preferably in the morning or at least 1 hour prior to meal. The capsules should be swallowed whole with water and not to be opened, chewed or crushed.
Pediatric patients
Pansa DSR Capsules are not recommended for use in children.
4.3 Contraindications
- Patients with known hypersensitivity to Pantoprazole or to any substituted Benzimidazole derivative or to Domperidone or to any component of the formulation.
- In patients receiving Rilpivirine-containing products.
- Prolactin-releasing pituitary tumor (prolactinoma).
- In patients with gastrointestinal hemorrhage, mechanical obstruction or perforation (i.e. when stimulation of the gastric motility could be harmful).
- In patients with moderate or severe hepatic impairment.
- In patients who have known existing prolongation of cardiac conduction intervals, particularly QTc.
- Patients with significant electrolyte disturbances (hypokalemia, hyperkalemia, hypomagnesemia) or underlying cardiac disease such as congestive heart failure (CHF).
- Co-administration with QT-prolonging drugs.
- Co-administration with potent CYP3A4 inhibitors.
4.4 Special warnings and precautions for use
Pantoprazole
Presence of gastric malignancy
In adults, symptomatic response to therapy with Pantoprazole does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing in adult patients who have a suboptimal response or an early symptomatic relapse after completing treatment with a Pantoprazole.
Acute interstitial nephritis
Acute interstitial nephritis has been observed in patients taking Pantoprazole. Acute interstitial nephritis may occur at any point during Pantoprazole therapy and is generally attributed to an idiopathic hypersensitivity reaction. Discontinue Pantoprazole if acute interstitial nephritis develops.
Clostridium difficile-associated diarrhea (CDAD)
Published observational studies suggest that Pantoprazole therapy may be associated with an increased risk of CDAD, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve.
Risk of bone fractures
Proton pump inhibitors (PPIs), especially if used in high doses and over long durations (> 1 year), may modestly increase the risk of hip, wrist and spine fracture, predominantly in the elderly or in presence of other recognized risk factors. Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk of osteoporosis should receive care according to current clinical guidelines and they should have an adequate intake of Vitamin D and Calcium.
Cutaneous and systemic lupus erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including Pantoprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. The occurrence of CLE with previous PPI treatment may increase the risk of CLE with other PPIs. Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. The majority of patients presented with rash. If lesions occur, especially in sun-exposed areas of the skin, and if accompanied by arthralgia, the patient should seek medical help promptly and Pantoprazole therapy should be stopped immediately. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks.
Cyanocobalamin (Vitamin B12) deficiency
Generally, daily treatment with any acid-suppressing medication over a long period of time (e.g. longer than 3 years) may lead to malabsorption of Vitamin B12 caused by hypo- or achlorhydria. Rare reports of Cyanocobalamin deficiency occurring with acidsuppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with Cyanocobalamin deficiency are observed.
Hypomagnesemia
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least 3 months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI. For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g. diuretics), monitoring of magnesium levels prior to initiation of PPI treatment and periodically thereafter should be considered.
Domperidone
Cardiovascular effects
Domperidone has been associated with prolongation of the QT interval on the electrocardiogram. During post-marketing surveillance, there have been very rare cases of QT prolongation and torsades de pointes in patients taking Domperidone. These reports included patients with confounding risk factors, electrolyte abnormalities and concomitant treatment which may have been contributing factors.
Epidemiological studies showed that Domperidone was associated with an increased risk of serious ventricular arrhythmias or sudden cardiac death. A higher risk was observed in patients older than 60 years, patients taking daily doses greater than 30 mg and patients concurrently taking QT-prolongation drugs or CYP3A4 inhibitors.
Domperidone is contraindicated in patients with known existing prolongation of cardiac conduction intervals, particularly QTc, in patients with significant electrolyte disturbances (hypokalaemia, hyperkalaemia, hypomagnesaemia), or bradycardia, or in patients with underlying cardiac diseases such as congestive heart failure (CHF) due to increased risk of ventricular arrhythmia. Electrolyte disturbances or bradycardia are known to be conditions increasing the proarrythmic risk. Treatment with Domperidone should be stopped if signs or symptoms occur that may be associated with cardiac arrhythmia, and the patients should consult their physician. Patients should be advised to promptly report any cardiac symptoms.
Use with Apomorphine
Domperidone is contraindicated with QT prolonging drugs including Apomorphine, unless the benefit of the co-administration with Apomorphine outweighs the risks.
Use in infants and children
Although neurological side effects are rare, the risk of neurological side effects are higher in young children since metabolic
functions and the blood-brain barrier are not fully developed in the first months of life. Overdosing may cause extrapyramidal
symptoms in children, but other causes should be taken into consideration.
4.5 Interaction with other medicinal products and other forms of interaction
Pantoprazole
Antiretroviral drugs
The effect of PPIs on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
- Decreased exposure of some antiretroviral drugs (e.g. Rilpivirine, Atazanavir and Nelfinavir) when used concomitantly with Pantoprazole may reduce antiviral effect and promote the development of drug resistance. Concomitant use of Rilpivirine containing products with Pantoprazole is contraindicated. Also, concomitant use of nelfinavir with Pantoprazole should be avoided.
- Increased exposure of other antiretroviral drugs (e.g. Saquinavir) when used concomitantly with Pantoprazole may increase toxicity
- There are other antiretroviral drugs which do not result in clinically relevant interactions with Pantoprazole.
Coumarin anticoagulants / Warfarin
There has been post-marketing reports of increased international normalized ratio (INR) and prothrombin time in patients receiving PPIs, including Pantoprazole and Warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Monitor INR and prothrombin time and adjust the dose of Warfarin if needed, to maintain the target INR range.
Clopidogrel
Concomitant administration of Pantoprazole and Clopidogrel in healthy subjects had no clinically important effect on exposure to the active metabolite of Clopidogrel or Clopidogrel-induced platelet inhibition. No dose adjustment of Clopidogrel is necessary when administered with an approved dose of Pantoprazole.
Methotrexate
Literature suggests that concomitant use of PPIs with Methotrexate (primarily at high dose) may elevate and prolong serum levels of Methotrexate and/or its metabolite, possibly leading to Methotrexate toxicities. A temporary withdrawal of Pantoprazole therapy may be considered in some patients receiving high-dose of Methotrexate.
Drugs for which gastric pH can affect bioavailability (iron salts, Erlotinib, Dasatinib, Nilotinib, Mycophenolate Mofetil, and Ketoconazole)
Pantoprazole causes long-lasting inhibition of gastric acid secretion. Therefore, Pantoprazole may reduce absorption of other drugs where gastric pH is an important determinant of their bioavailability.
Mycophenolate Mofetil (MMF)
Co-administration of Pantoprazole Sodium in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, Mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving Pantoprazole therapy and MMF. Use Pantoprazole with caution in transplant patients receiving MMF.
Drug / laboratory tests interactions
False positive urine tests for THC
There have been reports of false positive urine screening tests for Tetrahydrocannabinol (THC) in patients receiving PPIs, including Pantoprazole. An alternative confirmatory method should be considered to verify positive results.
Increased Chromogranin A (CgA) level
Increase in CgA may interfere with investigations for neuroendocrine tumours. To avoid this interference, Pantoprazole treatment should be stopped for at least 5 days before CgA measurements. If CgA and gastrin levels have not returned to reference range after initial measurement, measurements should be repeated 14 days after cessation of Pantoprazole treatment.
Domperidone
The main metabolic pathway of Domperidone is through CYP3A4. In vitro data suggest that the concomitant use of drugs that significantly inhibit this enzyme may result in increased plasma levels of Domperidone. There is increased risk of occurrence of QT-interval prolongation, due to pharmacodynamic and/or pharmacokinetic interactions.
Concomitant use of the following drugs is contraindicated.
A. QTc-prolonging medicinal products
- Anti-arrhythmic class IA (e.g. Disopyramide, Hydroquinidine, Quinidine).
- Anti-arrhythmic class III (e.g. Amiodarone, Dofetilide, Dronedarone, Ibutilide, Sotalol).
- Certain antipsychotics (e.g. Haloperidol, Pimozide, Sertindole). • Certain antidepressants (e.g. Citalopram, Escitalopram).
- Certain antibiotics (e.g. Erythromycin, Levofloxacin, Moxifloxacin, Spiramycin).
- Certain antifungal agents (e.g. Pentamidine).
- Certain antimalarial agents (e.g. Halofantrine, Lumefantrine).
- Certain gastrointestinal medicines (e.g. Cisapride, Dolasetron, Prucalopride).
- Certain antihistaminic (e.g. Mequitazine, Mizolastine).
- Certain medicines used in cancer (e.g. Toremifene, Vandetanib, Vincamine).
- Other medicines (e.g. Bepridil, Diphemanil, Methadone).
B. Potent CYP3A4 inhibitors (regardless of their QT prolonging effects)
- Protease inhibitors.
- Systemic azole antifungals.
- Some macrolides (e.g. Erythromycin, Clarithromycin and Telithromycin).
Concomitant use of the following drugs is not recommended.
- Moderate CYP3A4 inhibitors (e.g. Diltiazem, Verapamil and some macrolides).
Concomitant use of the following drugs requires caution.
- Caution with bradycardia and hypokalaemia-inducing drugs, as well as with the following macrolides involved in QT-interval prolongation : Azithromycin and Roxithromycin.
Ketoconazole / Erythromycin and QTc prolongation
Separate in vivo pharmacokinetic / pharmacodynamic interaction studies with oral Ketoconazole or oral erythromycin in healthy subjects confirmed a marked inhibition of Domperidone's CYP3A4 mediated first pass metabolism by these drugs (as both of these drugs significantly inhibit CYP3A4 enzyme). Both the Cmax and AUC of Domperidone at steady state were increased approximately three-fold in each of these interaction studies. In these studies, concomitant use of Domperidone and Ketoconazole or Erythromycin resulted in increase in QTc, over the observation period.
4.6 Use in special populations
Pregnancy
Pansa DSR capsule should be used during pregnancy only if the potential benefit justifies the possible risk to the fetus.
Breast-feeding
Pansa DSR capsules should not be used during breast feeding. Accordingly, a decision should be made whether to discontinue nursing or to discontinue / abstain from therapy, taking into account the benefit of the drug to the mother.
4.7 Effects on ability to drive and use machines
Both, Pantoprazole and Domperidone have no or negligible influence on the ability to drive and use machines. However, adverse reactions such as dizziness and visual disturbances may occasionally occur in patients on PPIs drug therapy. If affected, patients should not drive or use machines.
4.8 Undesirable effects
Pantoprazole
Clinical trials experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug may not reflect the rates observed in clinical practice. Common adverse reactions reported with Pantoprazole therapy in clinical trials with frequency > 2% include : Headache, diarrhoea, nausea, abdominal pain, vomiting, flatulence, dizziness, and arthralgia. Additional adverse reactions reported in clinical trials with a frequency of ≤ 2% include : Body as a whole :
Allergic reaction, pyrexia, photosensitivity reaction, facial edema
Gastrointestinal : Constipation, dry mouth, hepatitis
Hematologic : Leukopenia, thrombocytopenia
Metabolic / nutritional : Elevated creatine kinase (CK), generalized edema, elevated triglycerides, elevated liver enzymes
Musculoskeletal : Myalgia
Nervous : Depression, vertigo
Skin and appendages : Urticaria, rash, pruritus
Special senses : Blurred vision
Post-marketing experience
Acute kidney injury as an adverse drug reaction reported with the use of proton pump inhibitors. The following adverse reactions have been identified during post-approval use of Pantoprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders and administration conditions : Asthenia, fatigue, malaise
Hematologic : Pancytopenia, agranulocytosis
Hepatobiliary disorders : Hepatocellular damage leading to jaundice and hepatic failure
Immune system disorders : Anaphylaxis (including anaphylactic shock), SLE
Infections and infestations : Clostridium difficile-associated diarrhea
Investigations : Weight changes
Metabolism and nutritional disorders : Hyponatremia, hypomagnesemia
Musculoskeletal disorders : Rhabdomyolysis, bone fracture
Nervous system : Ageusia, dysgeusia
Psychiatric disorders : Hallucination, confusion, insomnia, somnolence
Renal and urinary disorders : Interstitial nephritis
Skin and subcutaneous tissue disorders : Severe dermatologic reactions, including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN), angioedema (Quincke’s edema) and CLE
Domperidone
Central nervous system : As the pituitary gland is outside the blood-brain barrier, Domperidone may cause an increase in prolactin levels. In rare cases this hyperprolactinaemia may lead to neuro-endocrinological side effects such as galactorrhoea, gynaecomastia and amenorrhoea.
Extrapyramidal side effects are very rare in neonates and infants, and exceptional in adults. These side effects reverse spontaneously and completely as soon as the treatment is stopped. Other central nervous system-related effects of convulsion, agitation and somnolence also are very rare and primarily reported in infants and children.
General disorders
Uncommon : Asthenia.
Immune system disorder
Not known : Anaphylactic reactions including anaphylactic shock and angioedema
Psychiatric disorders
Uncommon : Anxiety, loss of libido;
Not known : Agitation, nervousness
Nervous system disorders
Uncommon : Somnolence, headache;
Not known : Extrapyramidal disorder, convulsions
Eye disorders Not known : Oculogyric crisis Cardiac disorders
Not known : Ventricular arrhythmias, QTc prolongation, Torsade de Pointes, sudden cardiac death. Gastrointestinal disorders
Common : Dry mouth;
Uncommon : Diarrhea Skin and subcutaneous tissue disorders
Uncommon : Rash, pruritus;
Not known : Urticaria, angioedema Reproductive system and breast disorders
Uncommon : Breast pain, breast tenderness, galactorrhoea;
Not known : Gynaecomastia, amenorrhoea
Renal and urinary disorders
Not known : Urinary retention Investigations
Not known : Abnormal liver function test, increased blood prolactin
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
Website : https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Symptoms
Symptoms of overdose are agitation, altered consciousness, convulsions, disorientation, somnolence, and extrapyramidal reactions.
Treatment
There is no specific antidote, but in the event of overdose, gastric lavage as well as the administration of activated charcoal, may be useful. Close medical supervision and supportive therapy is recommended. Anticholinergic, Antiparkinson drugs may be helpful in controlling the extrapyramidal reactions.
5.0 Pharmacological properties
5.1 Mechanism of action
Pantoprazole
Pantoprazole is a proton pump inhibitor (PPI) class of anti-secretory agent. Pantoprazole is a lipophilic weak base that crosses the parietal cell membrane and enters the acidic parietal cell canaliculus where it becomes protonated, producing the active metabolite sulfenamide. Sulfenamide forms an irreversible covalent bond with two sites of the H+/K+-ATPase enzyme located on the gastric parietal cell. Thus, Pantoprazole suppress the final step in gastric acid (Hydrochloric Acid - HCl) production by covalently binding to the H+/K+-ATPase enzyme (also called as proton pump) system at the secretory surface of the gastric parietal cell. This effect leads to inhibition of both basal and stimulated gastric acid secretion, irrespective of the stimulus. The binding to the H+/K+-ATPase results in duration of anti-secretory effect that persists longer than 24 hours.
Domperidone
Domperidone is a dopamine receptor (D2) antagonist. Domperidone act predominantly on peripheral dopamine receptors and produces anti-emetic and gastro-kinetic effects. Domperidone does not readily cross the blood-brain barrier (BBB). Thus, in Domperidone users, especially in adults, extrapyramidal side effects are very rare (unlike metoclopramide). Anti-emetic effect of Domperidone is due to a combination of peripheral (gastro-kinetic) effects and antagonism of dopamine receptors in the chemoreceptor trigger zone (CTZ), which lies outside the BBB in the area postrema. Oral Domperidone also increases lower esophageal sphincter (LES) pressure, thus, improve antroduodenal motility and accelerate gastric emptying. There is no effect on gastric secretion.
5.2 Pharmacodynamic properties
With a single oral dose of 20 to 80 mg of Pantoprazole, a dose-dependent decrease in gastric acid secretion occurs. Following the initial oral dose of 40 mg Pantoprazole, a 51% mean inhibition was achieved by 2.5 hours. With once-a-day dosing for 7 days, the mean inhibition was increased to 85%. Acid secretion had returned to normal within a week after the last dose of Pantoprazole; there was no evidence of rebound hypersecretion.
Pantoprazole reduces acidity in the stomach and thereby increases gastrin in proportion to the reduction in acidity. The increase in gastrin is reversible. Since Pantoprazole binds to the enzyme distal to the receptor level, it can inhibit hydrochloric acid secretion independently of stimulation by other substances (Acetylcholine, histamine and gastrin). The fasting gastrin values increase under Pantoprazole. On short-term use, in most cases they do not exceed the upper limit of normal. During long-term treatment, gastrin levels double in most cases.
Domperidone
Prokinetic effect : The prokinetic (gastro-kinetic) properties of Domperidone are related to its peripheral dopamine receptor blocking action.
Antiemetic effect : Domperidone produces antiemetic effect by blocking dopamine receptors (D2) peripherally. Inhibition of peripheral D2 receptor signaling prevents or relieves various GI symptoms, such as nausea and vomiting, and also relieves reflux and other symptoms associated with upper GI disorders.
5.3 Pharmacokinetic properties
Pantoprazole
Absorption
Like other PPIs, Pantoprazole is an acid-labile drug and therefore, administered orally in the form of gastro-resistant pellets. Absorption of Pantoprazole, therefore, begins in the intestine only after the pellets leave the stomach. After administration of a single or multiple oral doses of Pantoprazole 40 mg, the peak plasma concentration of Pantoprazole was achieved in approximately 2.5 hours, and Cmax was 2.5 mcg/ml. Peak serum concentration (Cmax) and area under the serum concentration time curve (AUC) increases in a dose-dependent manner (with dose range from 10 to 80 mg). Pantoprazole does not accumulate, and its pharmacokinetics are unaltered with multiple daily dosing. Pantoprazole undergoes little first-pass metabolism, resulting in an absolute bioavailability of approximately 77%.
Effect of antacid / food
Pantoprazole absorption is not affected by concomitant administration of antacids. Administration of Pantoprazole with food may delay its absorption up to 2 hours or longer; however, the Cmax and the extent of Pantoprazole absorption (AUC) are not altered. Thus, Pantoprazole may be taken without regard to timing of meals.
Distribution
The apparent volume of distribution of Pantoprazole is approximately 11 to 23.6 liters, distributing mainly in extracellular fluid.
The plasma protein binding of Pantoprazole is about 98%, primarily to albumin.
Metabolism
Pantoprazole is extensively metabolized in the liver through the cytochrome P450 (CYP) system. The main metabolic pathway is demethylation, by CYP2C19, with subsequent sulfation; other metabolic pathways include oxidation by CYP3A4. There is no evidence that any of the Pantoprazole metabolites have significant pharmacologic activity.
Excretion
Renal elimination represents the major route of excretion (about 80 %) for the metabolites of Pantoprazole, the rest is excreted with the faeces. There is no renal excretion of unchanged Pantoprazole. The main metabolite in both the serum and urine is desmethyl Pantoprazole which is conjugated with Sulphate. Following oral administration, the serum concentration of Pantoprazole declines biexponentially, with a terminal elimination half-life of approximately one hour.
Domperidone
Pharmacokinetics of Domperidone in sustained release formulation is not available. Conventional formulation of Domperidone (i.e. immediate release) has following pharmacokinetic properties :
Absorption
Domperidone is rapidly absorbed after oral administration, with peak plasma concentrations occurring at approximately 1 hour after dosing. The Cmax and AUC values of Domperidone increased proportionally with dose in the 10 mg to 20 mg dose range. The low absolute bioavailability of oral Domperidone (approximately 15%) is due to an extensive first-pass metabolism in the gut and liver.
Distribution
Oral Domperidone does not appear to accumulate or induce its own metabolism. The peak plasma concentration (Cmax) of 18 ng/ml to 21 ng/ml occurs 1.5 hours (Tmax) after the oral dose. Domperidone is 91 to 93% bound to plasma proteins. Distribution studies with Domperidone have shown wide tissue distribution, but low brain concentration.
Metabolism
Domperidone undergoes rapid and extensive hepatic metabolism by hydroxylation and N-dealkylation. In vitro metabolism experiments with diagnostic inhibitors revealed that CYP3A4 is a major form of cytochrome P-450 involved in the N-dealkylation of Domperidone, whereas CYP3A4, CYP1A2 and CYP2E1 are involved in Domperidone aromatic hydroxylation.
Excretion
After oral dose, Domperidone is excreted mainly by renal (31%) and biliary (66%) routes. The proportion of the drug excreted unchanged is small (10% of fecal excretion and approximately 1% of urinary excretion). The plasma half-life after a single oral dose is 7 to 9 hours in healthy subjects, but is prolonged in patients with severe renal insufficiency.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Pantoprazole
Carcinogenesis
In a 24-month carcinogenicity study, Sprague-Dawley rats were treated orally with Pantoprazole doses of 0.5 to 200 mg/kg/day, about 0.1 to 40 times the exposure on a body surface area basis of a 50 kg person dosed with 40 mg/day. In the gastric fundus, treatment with 0.5 to 200 mg/kg/day produced enterochromaffin-like (ECL) cell hyperplasia and benign and malignant neuroendocrine cell tumors in a dose-related manner. In the forestomach, treatment with 50 and 200 mg/kg/day (about 10 and 40 times the recommended human dose on a body surface area basis) produced benign squamous cell papillomas and malignant squamous cell carcinomas. Rare gastrointestinal tumors associated with Pantoprazole treatment included an adenocarcinoma of the duodenum with 50 mg/kg/day and benign polyps and adenocarcinomas of the gastric fundus with 200 mg/kg/day. In the liver, treatment with 0.5 to 200 mg/kg/day produced dose-related increases in the incidences of hepatocellular adenomas and carcinomas. In the thyroid gland, treatment with 200 mg/kg/day produced increased incidences of follicular cell adenomas and carcinomas for both male and female rats. In a 24-month carcinogenicity study, B6C3F1 mice were treated orally with doses of 5 to 150 mg/kg/day of Pantoprazole, 0.5 to 15 times the recommended human dose based on body surface area. In the liver, treatment with 150 mg/kg/day produced increased incidences of hepatocellular adenomas and carcinomas in female mice. Treatment with 5 to 150 mg/kg/day also produced gastric-fundic ECL cell hyperplasia. A 26-week p53 +/-transgenic mouse carcinogenicity study was not positive.
Domperidone
Safety margins in in vitro proarrhythmic models (isolated Langendorff perfused heart) exceeded the free plasma concentrations in humans at maximum daily dose (10 mg administered 3 times a day) by 9- up to 45-fold. In in vivo models the no effect levels for QTc prolongation in dogs and induction of arrhythmias in a rabbit model sensitized for torsade de 13 pointes exceeded the free plasma concentrations in humans at maximum daily dose (10 mg administered 3 times a day) by more than 22-fold and 435-fold, respectively. In the anesthetized guinea pig model following slow intravenous infusions, there were no effects on QTc at total plasma concentrations of 45.4 ng/ml, which are 3-fold higher than the total plasma levels in humans at maximum daily dose (10 mg administered 3 times a day). At a high, maternally toxic dose (more than 40 times the recommended human dose), teratogenic effects were seen in the rat. No teratogenicity was observed in mice and rabbits. Development abnormalities observed in rats at a high exposure. Risk of carcinogenicity, mutagenicity or sensitisation cannot be excluded.
7.0 Description
Each capsule of Pansa DSR contains 40 mg of Pantoprazole (in a gastro-resistant form) and 30 mg of Domperidone (in a prolonged release form) for oral administration in adults.
Pantoprazole
Pantoprazole Sodium is the Sodium salt form of Pantoprazole. Pantoprazole is a substituted Benzimidazole, proton pump inhibitor (PPI) class of anti-secretory agents which suppressesgastric acid production.
Molecular weight : 405.40 g/mol
Molecular formula : C16H14F2N3NaO4S
Chemical name : Sodium; 5-(difluoromethoxy)-2-[(3,4-dimethoxypyridin-2- yl) methylsulfinyl] benzimidazol-1-ide Domperidone Domperidone is a dopamine receptor antagonist drug with antiemetic and gastrokinetic properties. Domperidone is white or almost white powder which is slightly soluble in water. Molecular weight : 425.90 g/mol Molecular formula : C22H24ClN5O2 Chemical name : 6-chloro-3-[1-[3-(2-oxo-3H-benzimidazol-1-yl)propyl]piperidin-4-yl]-1H-benzimidazol-2-one.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
Alu-Alu blister strip of 10 capsules.
8.4 Storage and handling instructions
Store protected from moisture at a temperature not exceeding 25°C.
Keep out of reach of children.
9.0 Patient counseling information
- Instruct patients to take Pansa DSR Capsule exactly as prescribed by doctor. Do not change the dose or stop therapy without consulting to your doctor.
- Instruct patients to swallow Pansa DSR Capsule as a whole with water and not to open, chew or crush the capsules.
- If you miss a dose, take it as soon as possible. If it is almost time for your next dose, do not take the missed dose. Take the next dose at your regular time. Do not take 2 capsules per doses at the same time to make up for the missed dose.
- Pregnant women can use this medicine only if essential and in consultation with their doctor.
- Advise nursing mothers to avoid use of this medicine during lactation or not to breastfeed their infants while on drug therapy.
- This medicine is not recommended for use in children.
- Instruct patients not to share this medication with other people even though symptoms are similar. It may harm them.
- Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Pansa DSR Capsules and certain other medicines can interact with each other causing serious side effects.
12.0 Date of issue
04 July 2024
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1.What PANSA DSR is and what it is used for
2. What you need to know before you take PANSA DSR
3. How to take PANSA DSR
4. Possible side effects
5. How to store PANSA DSR
6. Contents of the pack and other information
1. What PANSA DSR is and what it is used for
Pansa DSR Capsule is a combination of two medicines: Domperidone and Pantoprazole. Domperidone is a prokinetic which works on the upper digestive tract to increase the movement of the stomach and intestines, allowing the food to move more easily through the stomach. Pantoprazole is a proton pump inhibitor (PPI) which works by reducing the amount of acid in the stomach which helps in the relief of acid-related indigestion and heartburn
PANSA DSR is used for the treatment of Gastroesophageal reflux disease (occurs when stomach acid repeatedly flows back into the tube connecting your mouth and stomach that is esophagus)
2. What you need to know before you take PANSA DSR
Do not take PANSA DSR if you are
- If you are allergic to pantoprazole, domperidone or to any of the other ingredients listed in the formulation
- If you are allergic to medicines containing other proton pump inhibitors.
- You have black, tarry bowel motions (stools) or notice blood in your bowel motions. This could be a sign of bleeding in the stomach or intestines
- You have a blockage or tear in your intestines
- You have a tumour of the pituitary gland called a prolactinoma.
- have a disorder known as phenylketonuria (a metabolic disorder) orodispersible tablets should not be used as they contain aspartamine
- if you have a moderate or severe liver disease
- if your ECG (electrocardiogram) shows a heart problem called "prolonged QT corrected interval"
- if you have or had a problem where your heart cannot pump the blood round your body as well as it should (condition called heart failure).
- if you have a problem that gives you a low level of potassium or magnesium, or a high level of potassium in your blood.
Do not take PANSA DSR tablets if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist before taking this medicine. If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before taking PANSA DSR tablet. Do this even if they have applied in the past.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before taking PANSA DSR
- If you have severe liver problems. Please tell your doctor if you ever had problems with your liver in the past. He will check your liver enzymes more frequently, especially when you are taking Pantoprazole as a long-term treatment. In the case of a rise of liver enzymes the treatment should be stopped.
- If you have reduced body stores or risk factors for reduced vitamin B12 and receive long-term treatment with pantoprazole. As with all acid reducing agents, pantoprazole may lead to a reduced absorption of vitamin B12.
- If you are taking HIV protease inhibitors such as atazanavir (for the treatment of HIV-infection) at the same time as pantoprazole, ask your doctor for specific advice.
- Taking a proton pump inhibitor like pantoprazole, especially over a period of more than one year, may slightly increase your risk of fracture in the hip, wrist or spine. Tell your doctor if you have osteoporosis or if you are taking corticosteroids (which can increase the risk of osteoporosis).
- If you are on Pantoprazole for more than three months it is possible that the levels of magnesium in your blood may fall. Low levels of magnesium can be seen as fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness, increased heart rate. If you get any of these symptoms, please tell your doctor promptly. Low levels of magnesium can also lead to a reduction in potassium or calcium levels in the blood. Your doctor may decide to perform regular blood tests to monitor your levels of magnesium.
- If you have ever had a skin reaction after treatment with a medicine similar to Pantoprazole that reduces stomach acid.
- If you get a rash on your skin, especially in areas exposed to the sun tell your doctor as soon as you can, as you may need to stop your treatment with Pantoprazole. Remember to also mention any other ill-effects like pain in your joints.
- if you are due to have a specific blood test (Chromogranin A).
- if you suffer from liver problems (liver function impairment or failure)
- if you suffer from kidney problems (kidney function impairment or failure).
It is advisable to ask your doctor for advice in case of prolonged treatment as you may need to take a lower dose or take this medicine less often, and your doctor may want to examine you regularly. Domperidone may be associated with an increased risk of heart rhythm disorder and cardiac arrest. This risk may be more likely in those over 60 years old or taking doses higher than 30mg per day. The risk also increases when domperidone is given together with some drugs. Tell your doctor or pharmacist if you are taking drugs to treat infection (fungal infections or bacterial infection) and/or if you have heart problems or AIDS/HIV. Domperidone should be used at the lowest effective dose. While taking domperidone, contact your doctor if you experience heart rhythm disorders such as palpitations, trouble breathing, loss of consciousness. Treatment with domperidone should be stopped.
Adolescents weighing less than 35 kg and children
PANSA DSR should not be given to adolescents 12 years of age and older weighing less than 35 kg, or in any children less than 12 years of age, as it is not effective in these age groups.
Tell your doctor immediately, before or after taking this medicine, if you notice any of the following
- An unintentional loss of weight
- Vomiting, particularly if repeated
- Vomiting blood; this may appear as dark coffee grounds in your vomit
- You notice blood in your stools; which may be black or tarry in appearance
- Difficulty in swallowing or pain when swallowing
- You look pale and feel weak (anaemia)
- Chest pain, Stomach pain, severe and/or persistent diarrhoea, because this medicine
- has been associated with a small increase in infectious diarrhoea.
Your doctor may decide that you need some tests to rule out malignant disease because pantoprazole also alleviates the symptoms of cancer and could cause delay in diagnosing it. If your symptoms continue in spite of your treatment, further investigations will be considered. If you take Pantoprazole on a long-term basis (longer than 1 year) your doctor will probably keep you under regular surveillance. You should report any new and exceptional symptoms and circumstances whenever you see your doctor.
Other medicines and PANSA DSR
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription. This is because Pantoprazole may influence the effectiveness of other medicines, so tell your doctor if you are taking:
- Medicines such as ketoconazole, itraconazole and posaconazole (used to treat fungal infections) or erlotinib (used for certain types of cancer) because Pantoprazole may stop these and other medicines from working properly.
- Warfarin and phenprocoumon, which affect the thickening, or thinning of the blood. You may need further checks.
- Medicines used to treat HIV-infection, such as atazanavir.
- Methotrexate (used to treat rheumatoid arthritis, psoriasis, and cancer) – if you are taking methotrexate your doctor may temporarily stop your Pantoprazole treatment because pantoprazole can increase levels of methotrexate in the blood.
- Fluvoxamine (used to treat depression and other psychiatric diseases) – if you are taking fluvoxamine your doctor may reduce the dose.
- Rifampicin (used to treat infections).
- St John’s wort (Hypericum perforatum) (used to treat mild depression).
Do not take Domperidone tablet if you are taking medicine to treat:
- fungal infections such as azole antifungals, specifically oral ketoconazole, fluconazole or voriconazole
- bacterial infections, specifically erythromycin, clarithromycin, telithromycin,moxifloxacin, pentamidine (these are antibiotics)
- heart problems or high blood pressure (e.g., amiodarone, dronedarone, quinidine, disopyramide, dofetilide, sotalol, diltiazem, verapamil)
- psychoses (e.g., haloperidol, pimozide, sertindole)
- depression (e.g., citalopram, escitalopram)
- gastro-intestinal disorders (e.g., cisapride, dolasetron, prucalopride)
- allergy (e.g., mequitazine, mizolastine)
- malaria (in particular halofantrine)
- AIDS/HIV (protease inhibitors)
- Hepatitis C (e.g., telaprevir)
- cancer (e.g., toremifene, vandetanib, vincamine)
- certain other medicines (e.g., bepridil, diphemanil, methadone)
Before you use Domperidone tablet and apomorphine, your doctor will ensure that you tolerate both medicines when used simultaneously. Ask your doctor or specialist for a personalised advice. Tell your doctor or pharmacist if you are taking drugs to treat infection, heart problems or AIDS/HIV. It is important to ask your doctor or pharmacist if Domperidone tablet is safe for you when you are taking any other medicines, including medicines obtained without prescription.
Pregnancy
Pantoprazole: There are no adequate and well-controlled studies in pregnant women.
Domperidone: There are limited post-marketing data of its use in pregnant women. A study in rats has shown reproductive toxicity at a high, maternally toxic dose.
Because animal reproduction studies are not always predictive of human response, PANSA DSR tablet should be used during pregnancy only if clearly needed.
Lactation
There is insufficient data on the excretion of pantoprazole in human milk but excretion into human milk has been reported.
Domperidone is excreted in human milk and breast-fed infants receive less than 0.1% of the maternal weight-adjusted dose. Caution should be exercised in case of QTc prolongation risk factor in breast-fed infants.
A decision should be made whether to discontinue breast-feeding or to discontinue/abstain from PANSA DSR therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the women.
Driving and using machines
PANSA DSR has no or negligible influence on the ability to drive and use machines. If you experience side effects like dizziness or disturbed vision, you should not drive or operate machines.
3. How to take PANSA DSR
One capsule daily. The capsule should not be chewed or crushed, and should be swallowed whole 1 hour before a meal with some water.
Pediatric
PANSA DSR tablets are not recommended in children below 12 years of age.
Hepatic impairment
No dosage adjustment is needed in patients with mild to severe hepatic impairment. In patients with severe liver impairment, the liver enzymes should be monitored regularly during treatment with pantoprazole, particularly on long-term use.
In the case of a rise of the liver enzymes, the treatment should be discontinued. Doses higher than 40 mg/day have not been studied in patients with hepatic impairment
Renal impairment
In subjects with renal insufficiency (creatinine clearance<30 ml/min/1.73m2) the elimination half-life of domperidone was increased from 7.4 to 20.8 hours. Since very little unchanged drug (approx. 1%) is excreted via the kidneys, it is unlikely that the dose of a single administration needs to be adjusted in patients with renal insufficiency. However, on repeated administration, the dosing frequency of PANSA DSR should be reduced to once or twice daily depending on severity of the impairment.
If you take more PANSA DSR than you should
Consult your doctor or pharmacist. There are no known symptoms of overdose.
If you forget to take PANSA DSR
Do not take a double dose to make up for a forgotten dose. Take your next, normal dose at the usual time.
If you stop taking PANSA DSR
Do not stop taking these tablets without first talking to your doctor or pharmacist.
4. Possible Side Effects
Pantoprazole
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you get any of the following side effects, stop taking these tablets and tell your doctor immediately, or contact the casualty department at your nearest hospital:
Serious allergic reactions (frequency rare: may affect up to 1 in 1,000 people): swelling of the tongue and/or throat, difficulty in swallowing, hives (nettle rash), difficulties in breathing, allergic facial swelling (Quincke’s oedema / angioedema), severe dizziness with very fast heartbeat and heavy sweating.
Serious skin conditions (frequency not known: frequency cannot be estimated from the available data): blistering of the skin and rapid deterioration of your general condition, erosion (including slight bleeding) of eyes, nose, mouth/lips or genitals (Stevens-Johnson-Syndrome, Lyell-Syndrome, Erythema multiforme), and sensitivity to light.
Other serious conditions (frequency not known: frequency cannot be estimated from the available data): yellowing of the skin or whites of the eyes (severe damage to liver cells, jaundice) or fever, rash, and enlarged kidneys sometimes with painful urination, and lower back pain (serious inflammation of the kidneys), possibly leading to kidney failure.
Other side effects are:
Common (may affect up to 1 in 10 people) Benign polyps in the stomach.
Uncommon (may affect up to 1 in 100 people) Headache; dizziness; diarrhoea; feeling sick, vomiting; bloating and flatulence (wind); constipation; dry mouth; abdominal pain and discomfort; skin rash, exanthema, eruption; itching; feeling weak, exhausted or generally unwell; sleep disorders; fracture in the hip, wrist or spine.
Rare (may affect up to 1 in 1,000 people) Distortion or complete lack of the sense of taste; disturbances in vision such as blurred vision; hives; pain in the joints; muscle pains; weight changes; raised body temperature; high fever; swelling of the extremities (peripheral oedema); allergic reactions; depression; breast enlargement in males.
Very Rare (may affect up to 1 in 10,000 people) Disorientation.
Not known (frequency cannot be estimated from the available data) Hallucination, confusion (especially in patients with a history of these symptoms); decreased sodium level in blood, decreased magnesium level in blood, feeling of tingling, prickling, pins and needles, burning sensation or numbness, rash, possibly with pain in the joints, inflammation in the large bowel, that causes persistent watery diarrhoea.
Side effects identified through blood tests:
Uncommon (may affect up to 1 in 100 people) an increase in liver enzymes.
Rare (may affect up to 1 in 1,000 people) an increase in bilirubin; increased fat levels in blood; sharp drop in circulating granular white blood cells, associated with high fever.
Very Rare (may affect up to 1 in 10,000 people) a reduction in the number of blood platelets, which may cause you to bleed or bruise more than normal; a reduction in the number of white blood cells, which may lead to more frequent infections; coexisting abnormal reduction in the number of red and white blood cells, as well as platelets.
Domperidone
Uncommon (may affect up to 1 in 100 people): Involuntary movements of the face or arms and legs, excessive trembling, excessive muscle stiffness or muscle spasm
Not known (frequency cannot be estimated from the available data):
- Seizures
- a type of reaction that may occur soon after administration and is recognised by skin rash, itching, shortness of breath, and/or a swollen face.
- a severe hypersensitivity reaction that may occur soon after administration that is characterised by hives, itching, flushing, fainting, and difficulty breathing among other possible symptoms.
- disorders of the cardiovascular system: heart rhythm disorders (rapid or irregular heart beat) have been reported; if this happens, you should stop the treatment immediately.
Domperidone may be associated with an increased risk of heart rhythm disorder and cardiac arrest. This risk may be more likely in those over 60 years old or taking doses higher than 30 mg per day. Domperidone should be used at the lowest effective dose. Stop treatment with Domperidone and contact your doctor immediately if you experience any of the unwanted events described above.
Other unwanted effects that have been observed with Domperidone are listed below:
Common (may affect up to 1 in 10 people): Dry mouth
Uncommon (may affect up to 1 in 100 people): anxiety, agitation, nervousness, loss of interest in sex or diminished interest in sex, headache, sleepiness, diarrhoea, rash, itchiness, hives, Painful or tender breasts, milk discharge from breasts, a general feeling of weakness, feeling dizzy
Not known (frequency cannot be estimated from the available data): Upward movement of the eyes, stopped menstrual periods in women, enlarged breasts in men, inability to urinate, changes in certain laboratory test results, restless legs syndrome (uncomfortable feeling, with an irresistible urge to move your legs, and sometimes arms and other parts of your body).
Some patients who have used Domperidone for conditions and dosages requiring medical oversight have experienced the following unwanted effects: Restlessness; swollen or enlarged breasts, unusual discharge from breasts, irregular menstrual periods in women, difficulty breastfeeding, depression, hypersensitivity.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store PANSA DSR
Store protected from light & moisture at a temperature not exceeding 30°C.
Keep out of reach of children.
Do not use the tablets after the expiry date is stated on the label. Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Zosa Junior gastro-resistant granules for oral suspension contains
Each hard gelatin capsule contains:
Pantoprazole Sodium IP
equivalent to Pantoprazole- 40 mg
(As Gastro-resistant pellets)
Colours : Lake of Sunset Yellow
Domperidone IP -30 mg
(As Prolonged-release pellets)
Colour : Indigo Carmine
Excipients q.s.
Approved colours used in the capsule shell.
Packaging
A blister strip of 10 capsules