Pansa IV Injection
Therapy Area
Gastrointestinal
1.0 Generic name
Pantoprazole for Injection IP 40 mg
2.0 Qualitative and quantitative composition
Each vial contains :
Pantoprazole Sodium IP (Sterile)
(As Lyophilized Bulk)
equivalent to Pantoprazole 40 mg
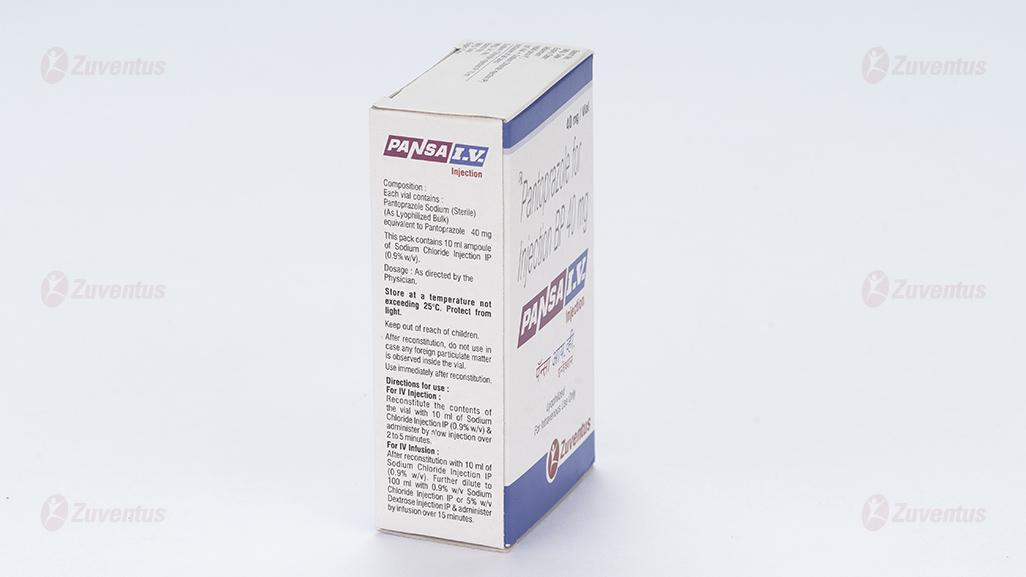
This pack contains 10 ml ampoule of Sodium Chloride Injection IP (0.9% w/v).
3.0 Dosage form and strength
Lyophilized Powder for solution for injection 40mg / Vial
4.0 Clinical particulars
4.1 Therapeutic indication
Duodenal ulcer, gastric ulcer, moderate and severe reflux oesophagitis.
4.2 Posology and method of administration
Pantoprazole Sodium IP is available in 40mg strength vial.
This medicine should be administered by a healthcare professional and under appropriate medical supervision.
Intravenous administration of Pansa IV is recommended only if oral administration is not appropriate. Data are available on intravenous use for up to 7 days. Therefore, as soon as oral therapy is possible, treatment with Pansa IV should be discontinued and 40 mg pantoprazole p.o. should be administered instead.
Gastric and duodenal ulcer, reflux oesophagitis : The recommended intravenous dose is one vial of Pansa IV (40 mg pantoprazole) per day.
In case a rapid acid control is required, a starting dose of 2 x 80 mg Pansa IV is sufficient to manage a decrease of acid output into the target range (<10 mEq/h) within one hour in the majority of patients.
Hepatic Impairment : A daily dose of 20 mg pantoprazole (half a vial of 40 mg pantoprazole) should not be exceeded in patients with severe liver impairment.
Renal Impairment : No dose adjustment is necessary in patients with impaired renal function.
Elderly : No dose adjustment is necessary in elderly patients.
Paediatric population : Pansa IV is not recommended for use in patients below 18 years of age.
For IV Injection : Reconstitute the contents of the vial with 10 ml of Sodium Chloride Injection IP (0.9% w/v) & administer by slow injection over 2 to 5 minutes.
For IV Infusion : After reconstitution with 10 ml of Sodium Chloride Injection IP (0.9% w/v). Further dilute to 100 ml with 0.9% w/v Sodium Chloride Injection IP or 5% w/v Dextrose Injection IP & administer by infusion over 15 minutes.
4.3 Contraindications
Hypersensitivity to the active substance, substituted benzimidazoles, or to any of the excipients listed in the formulation.
4.4 Special warnings and precautions for use
Gastric malignancy : Symptomatic response to pantoprazole may mask the symptoms of gastric malignancy and may delay diagnosis. In the presence of any alarm symptom (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis, anaemia or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded. Further investigation is to be considered if symptoms persist despite adequate treatment.
Hepatic impairment : In patients with severe liver impairment, the liver enzymes should be monitored during therapy. In the case of a rise of the liver enzymes, the treatment should be discontinued.
Co-administration with HIV protease inhibitors : Co-administration of pantoprazole is not recommended with HIV protease inhibitors for which absorption is dependent on acidic intragastric pH such as Atazanavir due to significant reduction in their bioavailability.
Gastrointestinal infections caused by bacteria : Treatment with Pansa IV may lead to a slightly increased risk of gastrointestinal infections caused by bacteria such as Salmonella and Campylobacter or C. difficile.
Hypomagnesaemia : Severe hypomagnesaemia has been reported in patients treated with PPIs like pantoprazole for at least three months, and in most cases for a year. Healthcare professionals should consider measuring magnesium levels before starting PPI treatment and periodically during treatment.
Bone fractures : Proton pump inhibitors, especially if used in high doses and over long durations (>1 year), may modestly increase the risk of hip, wrist and spine fracture, predominantly in the elderly or in presence of other recognised risk factors. Patients at risk of osteoporosis should receive care according to current clinical guidelines and they should have an adequate intake of vitamin D and calcium.
Subacute cutaneous lupus erythematosus (SCLE) : Proton pump inhibitors are associated with very infrequent cases of SCLE. If lesions occur, especially in sun-exposed areas of the skin, and if accompanied by arthralgia, the patient should seek medical help promptly and the health care professional should consider stopping Pansa IV. SCLE after previous treatment with a proton pump inhibitor may increase the risk of SCLE with other proton pump inhibitors.
Interference with laboratory tests : Increased Chromogranin A (CgA) level may interfere with investigations for neuroendocrine tumours. To avoid this interference, Pansa IV treatment should be stopped for at least 5 days before CgA measurements. If CgA and gastrin levels have not returned to reference range after initial measurement, measurements should be repeated 14 days after cessation of proton pump inhibitor treatment.
4.5 Drug interactions
Medicinal products with pH dependent absorption pharmacokinetics :
Because of profound and long lasting inhibition of gastric acid secretion, pantoprazole may interfere with the absorption of other medicinal products where gastric pH is an important determinant of oral bioavailability, e.g. some azole antifungals such as ketoconazole, itraconazole, posaconazole and other medicine such as erlotinib.
HIV protease inhibitors
Co-administration of pantoprazole is not recommended with HIV protease inhibitors for which absorption is dependent on acidic intragastric pH such as atazanavir due to significant reduction in their bioavailability. If the combination of HIV protease inhibtiors with a proton pump inhibitor is judged unavoidable, close clinical monitoring (e.g. virus load) is recommended. A pantoprazole dose of 20 mg per day should not be exceeded. Dosage of the HIV protease inhibitor may need to be adjusted.
Coumarin anticoagulants (phenprocoumon or warfarin)
Co-administration of pantoprazole with warfarin or phenprocoumon did not affect the pharmacokinetics of warfarin, phenoprocoumon or INR. However, there have been reports of increased INR and prothrombin time in patients receiving PPIs and war farin or phenoprocoumon concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding, and even death. Patients treated with pantoprazole and warfarin or phenprocoumon may need to be monitored for increase in INR and prothrombin time.
Methotrexate
Concomitant use of high dose methotrexate (e.g. 300 mg) and proton-pump inhibitors has been repor ted to increase methotrexate levels in some patients. Therefore, in settings where high-dose methotrexate is used, for example cancer and psoriasis, a temporary withdrawal of pantoprazole may need to be considered.
Other interactions studies
Pantoprazole is extensively metabolized in the liver via the cytochrome P450 enzyme system. The main metabolic pathway is demethylation by CYP2C19 and other metabolic pathways include oxidation by CYP3A4.
Medicinal products that inhibit or induce CYP2C19
Inhibitors of CYP2C19 such as fluvoxamine could increase the systemic exposure of pantoprazole. A dose reduction may be considered for patients treated long-term with high doses of pantoprazole, or those with hepatic impairment.
Enzyme inducers affecting CYP2C19 and CYP3A4 such as rifampicin and St John´s wort (Hypericum perforatum) may reduce the plasma concentrations of PPIs that are metabolized through these enzyme systems.
4.6 Use in special populations
Pregnancy
A moderate amount of data on pregnant women (between 300-1000 pregnancy outcomes) indicate no malformative or feto / neonatal toxicity of Pantoprazole 40 mg Powder for Solution for Injection. As a precautionary measure, it is preferable to avoid the use of Pantoprazole 40 mg Powder for Solution for Injection during pregnancy.
Breast-feeding
There is insufficient information on the excretion of pantoprazole in human milk but excretion into human milk has been reported. A risk to the newborns/infants cannot be excluded. Therefore, a decision on whether to discontinue breast-feeding or to discontinue/abstain from Pansa IV therapy should take into account the benefit of breast-feeding for the child, and the benefit for the woman.
Fertility
There was no evidence of impaired fertility following the administration of pantoprazole in animal studies.
4.7 Effects on the ability to drive and use machines
Pantoprazole has no or negligible influence on the ability to drive and use machines.
Adverse drug reactions such as dizziness and visual disturbances may occur. If affected, patients should not drive or operate machines.
4.8 Undesirable effects
Adverse drug reaction reports show that Proton Pump Inhibitors like Pantoprazole, are associated with acute kidney injury.
The table below lists adverse reactions reported with pantoprazole, ranked under the following frequency classification : Very common (≥ 1/10); common (≥ 1/100 to <1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000), not known (cannot be estimated from the available data).
| Common | Fundic gland polyps (benign), Injection site thrombophlebitis |
| Uncommon | Sleep disorders, headache, dizziness, diarrhea, nausea/vomiting, abdominal distension and bloating, constipation, dry mouth, abdominal pain and discomfort, liver enzymes increased (transaminases, γ-GT), rash/ exanthema/eruption, pruritus, fracture of the hip, wrist or spine, asthenia, fatigue and malaise |
| Rare | Agranulocytosis, Hypersensitivity (including anaphylactic reactions and anaphylactic shock), hyperlipidaemi as and lipid increases (triglycerides, cholesterol), weight changes, depression, taste disorders, disturbances in vision/blurred vision, bilirubin increased, urticaria, angioedema, arthralgia, myalgia, gynaecomastia, Body temperature increased, oedema peripheral. |
| Very Rare | Thrombocytopenia, leukopenia, pancytopenia, disorientation |
| Not known | Hyponatraemia, hypomagnesaemia, hypocalcaemia in association with hypomagnesemia, hypokalaemia, hallucination, confusion (especially in pre-disposed patients as well as the aggravation of these symptoms in case of pre-existence), paraesthesia, microscopic colitis, hepatocellular injury, Jaundice, hepatocellular failure, Stevens-Johnson syndrome, lyell syndrome; Erythema multiforme, photosensitivity, muscle spasm as a consequence of electrolyte disturbances, Interstitial nephritis (with possible progression to renal failure), subacute cutaneous lupus erythematosus |
Reporting of side effects
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
Website : http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
There are no known symptoms of overdose in man. Systemic exposure with up to 240 mg administered intravenously over 2 minutes were well tolerated. As pantoprazole is extensively protein bound, it is not readily dialysable. In the case of an overdose with clinical signs of intoxication, apart from symptomatic and supportive treatment, no specific therapeutic recommendations can be made.
5.0 Pharmacological properties
5.1 Mechanism of action
Pantoprazole is a substituted benzimidazole which inhibits the secretion of hydrochloric acid in the stomach by specific blockade of the proton pumps of the parietal cells.
Pantoprazole is converted to its active form in the acidic environment in the parietal cells where it inhibits the H+, K+-ATPase enzyme, i.e. the final stage in the production of hydrochloric acid in the stomach. The inhibition is dose-dependent and affects both basal and stimulated acid secretion. In most patients, freedom from symptoms is achieved within 2 weeks. As with other proton pump inhibitors and H2 receptor inhibitors, treatment with pantoprazole reduces acidity in the stomach and thereby increases gastrin in proportion to the reduction in acidity. The increase in gastrin is reversible. Since pantoprazole binds to the enzyme distal to the cell receptor level, it can inhibit hydrochloric acid secretion independently of stimulation by other substances (acetylcholine, histamine, gastrin). The effect is the same whether the product is given orally or intravenously.
5.2 Pharmacodynamic properties
The fasting gastrin values increase under pantoprazole. On short-term use, in most cases they do not exceed the upper limit of normal. During long-term treatment, gastrin levels double in most cases. An excessive increase, however, occurs only in isolated cases. As a result, a mild to moderate increase in the number of specific endocrine (ECL) cells in the stomach is observed in a minority of cases during long-term treatment (simple to adenomatoid hyperplasia). However, according to the studies conducted so far, the formation of carcinoid precursors (atypical hyperplasia) or gastric carcinoids as were found in animal experiments have not been observed in humans.
During treatment with antisecretory medicinal products, serum gastrin increases in response to the decreased acid secretion. Also CgA increases due to decreased gastric acidity. The increased CgA level may interfere with investigations for neuroendocrine tumours. Available published evidence suggests that proton pump inhibitors should be discontinued between 5 days and 2 weeks prior to CgA measurements. This is to allow CgA levels that might be spuriously elevated following PPI treatment to return to reference range. An influence of a long term treatment with pantoprazole exceeding one year cannot be completely ruled out on endocrine parameters of the thyroid according to results in animal studies.
5.3 Pharmacokinetic properties
Pharmacokinetics do not vary after single or repeated administration. In the dose range of 10 to 80 mg, the plasma kinetics of pantoprazole are linear after both oral and intravenous administration.
Distribution
Pantoprazole's serum protein binding is about 98%. Volume of distribution is about 0.15 l/kg.
Biotransformation
The substance is almost exclusively metabolized in the liver. The main metabolic pathway is demethylation by CYP2C19 with subsequent sulphate conjugation, other metabolic pathways include oxidation by CYP3A4.
Elimination
Terminal half-life is about 1 hour and clearance is about 0.1 l/h/kg. There were a few cases of subjects with delayed elimination. Because of the specific binding of pantoprazole to the proton pumps of the parietal cell the elimination half-life does not correlate with the much longer duration of action (inhibition of acid secretion).
Renal elimination represents the major route of excretion (about 80%) for the metabolites of pantoprazole, the rest is excreted with the faeces. The main metabolite in both the serum and urine is desmethylpantoprazole which is conjugated with sulphate. The half-life of the main metabolite (about 1.5 hours) is not much longer than that of pantoprazole.
6.0 Nonclinical Properties
6.1 Animal Toxicology or Pharmacology
Nonclinical data reveal no special hazard to humans based on conventional studies of safety pharmacology, repeated dose toxicity and genotoxicity. Studies also revealed no evidence of impaired fertility or teratogenic effects.
A slight increase of neoplastic changes of the thyroid was observed in the group of rats receiving the highest dose (200 mg/kg). As the therapeutic dose in man is low, no harmful effects on the thyroid glands are expected.
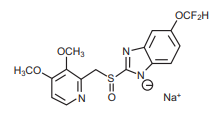
7.0 Description
The active ingredient, Pantoprazole Sodium IP, a PPI, is a substituted benzimidazole, sodium 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl] sulfinyl]- 1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C16H14F2N3NaO4S, with a molecular weight of 405.4.
The structural formula is :
8.0 Pharmaceutical particulars
8.1 Incompatibilities
This medicinal product must not be mixed with other medicinal products except for :
- 0.9% Sodium chloride solution
- Glucose 5% solution
It should not be prepared or mixed with solvents other than those stated above.
8.2 Shelf-life
Refer on the pack.
8.3 Packaging Information
A vial of 40 mg with Sodium Chloride Injection IP 10 ml.
8.4 Storage and handling instructions
Store protected from light & moisture at a temperature not exceeding 30°C.
Keep out of reach of children.
After reconstitution, do not use in case any foreign particulate matter is observed inside the vial.
9.0 Patient counselling information
Adverse reactions
Advise patients to report to their healthcare provider if they experience any signs or symptoms consistent with:
- Injection Site Reactions
- Acute Tubulointerstitial Nephritis
- Clostridium difficile- Associated Diarrhea
- Bone Fracture
- Severe Cutaneous Adverse Reactions
- Cutaneous and Systemic Lupus Erythematosus
- Hepatic Effects
- Hypomagnesemia and Mineral Metabolism
Drug interactions
Advise patients to report to their healthcare provider before they start treatment with any of the following:
- Rilpivirine-containing products
- High-dose methotrexate
Pregnancy
Advise a pregnant woman of the potential risk to a fetus. Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy.
12.0 Date of revision
05 June 2024
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What PANSA®IV is and what it is used for
- What you need to know before you take PANSA®IV
- How to take PANSA®IV
- Possible side effects
- How to store PANSA®IV
- Contents of the pack and other information
1. What PANSA®IV is and what it is used for
PANSA®IV (Pantoprazole) injection, for IV use, contains the active substance Pantoprazole. Pantoprazole is a selective “proton pump inhibitor”, a medicine which reduces the amount of acid produced in your stomach. It is used for treating acid-related diseases of the stomach and intestine.
This preparation is injected into a vein and will only be given to you if your doctor thinks pantoprazole injections are more suitable for you at the moment than pantoprazole tablets. Tablets will replace your injections as soon as your doctor sees fit.
PANSA®IV is used for treating adults for
- Reflux oesophagitis. An inflammation of your oesophagus (the tube which connects your throat to your stomach) accompanied by the regurgitation of stomach acid.
- Stomach and duodenal ulcers.
2. What you need to know before you take PANSA®IV
Do not use PANSA®IV
- If you are allergic to Pantoprazole or any of the other ingredients of this medicine
- If you are allergic to medicines containing other proton pump inhibitors.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using PANSA®IV
- If you have severe liver problems. Please tell your doctor if you have ever had problems with your liver in the past. Your doctor will check your liver enzymes more frequently. In the case of a rise of liver enzymes the treatment should be stopped.
- If you are taking HIV protease inhibitors such as atazanavir (for the treatment of HIV-infection) at the same time as pantoprazole, ask your doctor for specific advice.
- Taking a proton pump inhibitor like pantoprazole, especially over a period of more than one year, may slightly increase your risk of fracture in the hip, wrist or spine. Tell your doctor if you have osteoporosis (reduced bone density) or if you have been told that you are at risk of getting osteoporosis (for example, if you are taking steroids).
- If you are on PANSA®IV for more than three-months it is possible that the levels of magnesium in your blood may fall. Low levels of magnesium can be seen as fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness or increased heart rate. If you get any of these symptoms, please tell your doctor promptly. Low levels of magnesium can also lead to a reduction in potassium or calcium levels in the blood. Your doctor may decide to perform regular blood tests to monitor your levels of magnesium.
- If you have ever had a skin reaction after treatment with a medicine similar to PANSA®IV that reduces stomach acid.
- If you get a rash on your skin, especially in areas exposed to the sun tell your doctor as soon as you can, as you may need to stop your treatment with PANSA®IV. Remember to also mention any other ill-effects like pain in your joints.
- Serious skin reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS) and erythema multiforme, have been reported in association with pantoprazole treatment. Stop using pantoprazole and seek medical attention immediately if you notice any of the symptoms related to these serious skin reactions.
- If you are due to have a specific blood test (Chromogranin A)
Tell your doctor immediately, before or after taking this medicine, if you notice any of the following symptoms, which could be a sign of another, more serious, disease:
- an unintentional loss of weight
- vomiting, particularly if repeated
- vomiting blood; this may appear as dark coffee grounds in your vomit
- you notice blood in your stools; which may be black or tarry in appearance
- difficulty in swallowing or pain when swallowing
- you look pale and feel weak (anaemia)
- chest pain
- stomach pain
- severe and/or persistent diarrhoea, because this medicine has been associated with a small increase in infectious diarrhoea
Your doctor may decide that you need some tests to rule out malignant disease because pantoprazole also alleviates the symptoms of cancer and could cause delay in diagnosing it. If your symptoms continue in spite of your treatment, further investigations will be considered.
Children and adolescents
PANSA®IV is not recommended for use in children as it has not been proven to work in children below 18 years of age.
Other medicines and PANSA®IV
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
This is because PANSA®IV may influence the effectiveness of other medicines, so tell your doctor if you are taking:
- Medicines such as ketoconazole, itraconazole and posaconazole (used to treat fungal infections) or erlotinib (used for certain types of cancer) because PANSA®IV may stop these and other medicines from working properly.
- Warfarin and phenprocoumon, which affect the thickening, or thinning of the blood. You may need further checks.
- Medicines used to treat HIV-infection, such as atazanavir.
- Methotrexate (used to treat rheumatoid arthritis, psoriasis, and cancer) – if you are taking methotrexate your doctor may temporarily stop your PANSA®IV treatment because pantoprazole can increase levels of methotrexate in the blood.
- Fluvoxamine (used to treat depression and other psychiatric diseases) – if you are taking fluvoxamine your doctor may reduce the dose.
- Rifampicin (used to treat infections).
- St John’s wort (Hypericum perforatum) (used to treat mild depression).
Talk to your doctor before taking pantoprazole if you are due to have a specific urine test (for THC; Tetrahydrocannabinol).
Pregnancy, breast-feeding and fertility
There are no adequate data from the use of pantoprazole in pregnant women. Excretion into human milk has been reported.
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You should use this medicine, only if your doctor considers the benefit for you greater than the potential risk for your unborn child or baby.
Driving and using machines
PANSA®IV has no or negligible influence on the ability to drive and use machines.
If you experience side effects like dizziness or disturbed vision, you should not drive or operate machines.
3. How to take PANSA IV injection
Your nurse or your doctor will administer the daily dose to you as an injection into a vein over a period of 2 - 15 minutes.
The recommended dose is:
Adults
For gastric ulcers, duodenal ulcers and reflux oesophagitis.
One vial (40 mg pantoprazole) a day.
Patients with liver problems
If you suffer from severe liver problems, the daily injection should be only 20 mg (half a vial).
Use in children and adolescents
These injections are not recommended for use in children and adolescents under 18 years.
If you use more PANSA®IV than you should
These doses are carefully checked by your nurse or your doctor so an overdose is extremely unlikely. There are no known symptoms of overdose.
If you have any further questions about the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
See a doctor immediately if you experience any of the following side effects.
The serious side effects of PANSA®IV are:
- Serious allergic reactions (frequency rare: may affect up to 1 in 1,000 people): swelling of the tongue and/or throat, difficulty in swallowing, hives (nettle rash), difficulties in breathing, allergic facial swelling (Quincke’s oedema / angioedema), severe dizziness with very fast heartbeat and heavy sweating.
- Serious skin conditions (frequency not known: frequency cannot be estimated from the available data): you may notice one or more of the following - blistering of the skin and rapid deterioration of your general condition, erosion (including slight bleeding) of eyes, nose, mouth/lips or genitals, or skin sensitivity/rash, particularly in areas of skin exposed to light/the sun. You may also have joint pain or flu-like symptoms, a fever, swollen glands (e.g. in the armpit) and blood tests may show changes in certain white blood cells or liver enzymes.
- reddish non-elevated, target-like or circular patches on the trunk, often with central blisters, skin peeling, ulcers of mouth, throat, nose, genitals and eyes. These serious skin rashes can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis).
- widespread rash, high body temperature and enlarged lymph nodes (DRESS syndrome or drug hypersensitivity syndrome).
- Other serious conditions (frequency not known: frequency cannot be estimated from the available data): yellowing of the skin or whites of the eyes (severe damage to liver cells, jaundice) or fever, rash, and enlarged kidneys sometimes with painful urination, and lower back pain (serious inflammation of the kidneys), possibly leading to kidney failure
Other side effects are
- Common (may affect up to 1 in 10 people)
Inflammation of the wall of the vein and blood clotting (thrombophlebitis) where the medicine is injected; benign polyps in the stomach.
- Uncommon (may affect up to 1 in 100 people)
Headache; dizziness; diarrhoea; feeling sick, vomiting; bloating and flatulence (wind); constipation; dry mouth; abdominal pain and discomfort; skin rash, exanthema, eruption; itching; feeling weak, exhausted or generally unwell; sleep disorders; fracture in the hip, wrist or spine.
- Rare (may affect up to 1 in 1,000 people)
Distortion or complete lack of the sense of taste; disturbances in vision such as blurred vision; hives; pain in the joints; muscle pains; weight changes; raised body temperature; high fever; swelling of the extremities (peripheral oedema); allergic reactions; depression; breast enlargement in males.
- Very Rare (may affect up to 1 in 10,000 people)
Disorientation.
- Not known (frequency cannot be estimated from the available data)
Hallucination, confusion (especially in patients with a history of these symptoms); feeling of tingling, prickling, pins and needles, burning sensation or numbness, rash, possibly with pain in the joints, inflammation in the large bowel, that causes persistent watery diarrhoea.
Side effects identified through blood tests:
- Uncommon (may affect up to 1 in 100 people): an increase in liver enzymes.
- Rare (may affect up to 1 in 1,000 people): an increase in bilirubin; increased fat levels in blood; sharp drop in circulating granular white blood cells, associated with high fever.
- Very Rare (may affect up to 1 in 10,000 people): a reduction in the number of blood platelets, which may cause you to bleed or bruise more than normal; a reduction in the number of white blood cells, which may lead to more frequent infections; coexisting abnormal reduction in the number of red and white blood cells, as well as platelets.
- Not known (frequency cannot be estimated from the available data): decreased level of sodium, magnesium, calcium or potassium in blood
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store PANSA®IV injection
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date, which is stated on the carton and the vial after EXP. The expiry date refers to the last day of that month.
Do not store above 30°C.
Keep the vial in the outer carton in order to protect it from light.
Do not use PANSA®IV if you notice that the visual appearance has changed (e.g., if cloudiness or precipitation is observed).
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment
6. Contents of the pack and other information
What PANSA®IV 40 contains The active substance is Pantoprazole. Each vial contains 40 mg of Pantoprazole (as pantoprazole sodium).