Pegclear
Therapy Area
Gastrointestinal
1.0 Generic Name
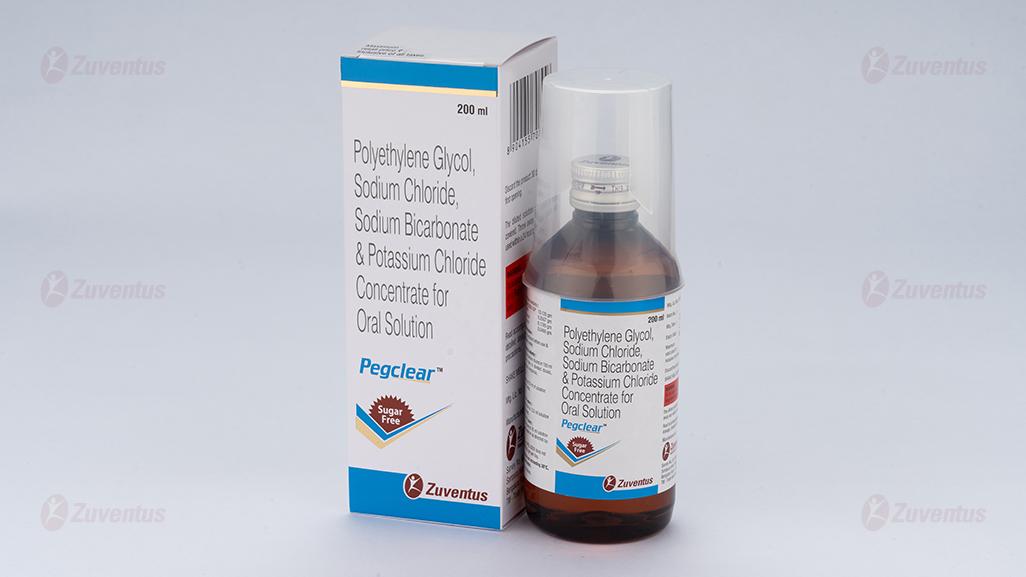
Pegclear Concentrate for Oral Solution
2.0 Qualitative and quantitative composition
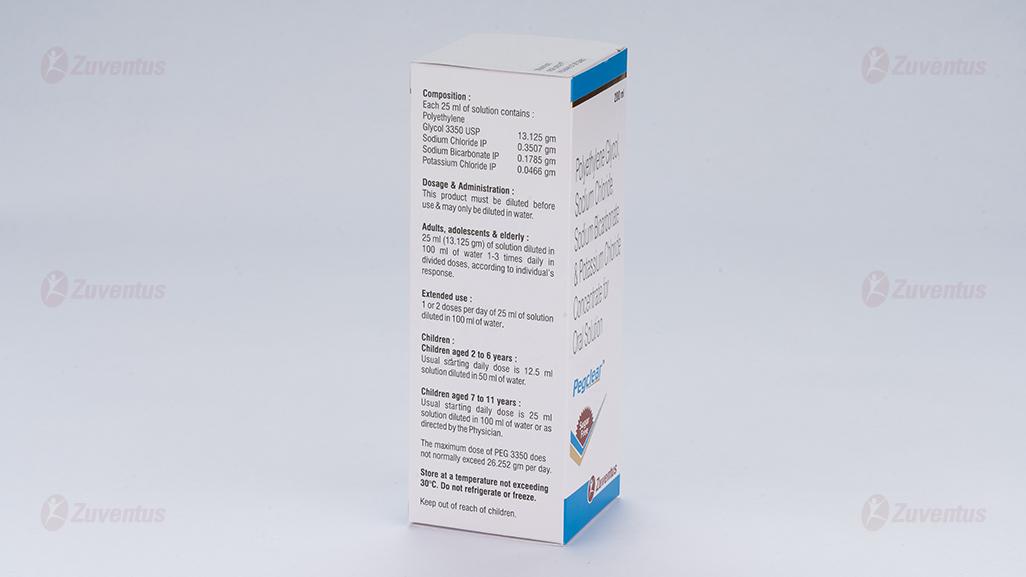
Each 25 ml contains:
Polyethylene Glycol 3350 USP 13.125 gm
Sodium Chloride IP 0.3507 gm
Sodium Bicarbonate IP 0.1785 gm
Potassium Chloride IP 0.0466 gm
3.0 Dosage form and strength
Concentrate for oral solution, clear and colourless liquid.
4.0 Clinical particulars
4.1. Therapeutic indication
For the treatment of chronic constipation.
For bowel cleansing prior to colonoscopy in adult patients only.
4.2. Posology and method of administration
Posology
A course of treatment for constipation with Pegclear should not normally exceed 2 weeks, although this can be repeated if required. As for all laxatives, prolonged use is not usually recommended. Extended use may be necessary in the case of patients with severe chronic or resistant constipation secondary to multiple sclerosis or parkinson's disease, or induced by regular constipating medication in particular opioids and antimuscarinics.
Adults, adolescents & elderly :
25 ml (13.125 gm) of solution diluted in 100 ml of water 1-3 times daily in divided doses, according to individual’s response. For extended use, the dose can be adjusted down to 1 or 2 doses per day of 25 ml of solution diluted in 100 ml of water.
Children :
2 to 6 years : usual starting daily dose is 12.5 ml solution diluted in 50 ml of water.
7 to 11 years : Usual starting daily dose is 25 ml solution diluted in 100 ml of water. The dose should be adjusted up or down as required to produce regular soft stools. If the dose needs increasing, this is best done every second day. The maximum dose of PEG 3350 does not normally exceed 26.252 gm per day.
Geriatric use
Can be administered to elderly patients in the same dosage as recommended for adults
Method of administration
Pegclear must not be taken undiluted and may only be diluted in water. Dilute required dose (volume) of Pegclear with water as recommended under dosage. Any unused solution should be discarded within 24 hours.
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients of the formulation; intestinal perforation or obstruction due to structural or functional disorder of the gut wall, ileus; severe inflammatory conditions of the intestinal tract, such as crohn's disease and ulcerative colitis; toxic megacolon.
4.4. Special warnings and precautions for use
If patients develop any symptoms indicating shifts of fluids/electrolytes like oedema, shortness of breath, increasing fatigue, dehydration, cardiac failure; PEG 3350 should be stopped immediately, electrolytes should be measured and any abnormality should be treated appropriately.
PEG 3350 increases gastro-intestinal transit rate which may transiently reduce absorption of other medicinal products. The sodium content should be taken into consideration when administering the product to patients on a controlled sodium diet. No dosage change is necessary for the treatment of constipation in patients with renal insufficiency.
4.5. Interaction with other medicinal products and other forms of interaction
Polyethylene glycol raises the solubility of medicinal products that are soluble in alcohol and relatively insoluble in water. PEG 3350 increases gastro-intestinal transit rate which may transiently reduce absorption of other medicinal products. There have been isolated reports of decreased efficacy with some concomitantly administered medicinal products, e.g. anti-epileptics.
4.6. Fertility, pregnancy and lactation
Pregnancy
There is limited data on the use of PEG 3350 in pregnant women. Studies in animals have shown indirect reproductive toxicity like reduction in fetal and placental weights, reduced fetal viability, increased limb and paw hyperflexion and abortions. Clinically, no effects during pregnancy are anticipated, since systemic exposure to PEG 3350 is negligible. PEG 3350 can be used during pregnancy.
Lactation
As systemic exposure of the breast-feeding woman to PEG 3350 is negligible, no effects on the breastfed newborn/infant are anticipated, and PEG 3350 can be used during breast-feeding.
Fertility
There are no data on the effects of PEG 3350 on fertility in humans. There were no effects on fertility in studies in male and female rats.
4.7. Effects on ability to drive and use machines
Pegclear concentrate for oral solution has no influence on the ability to drive and use machines.
4.8. Undesirable effects
Gastrointestinal tract related reactions occur most commonly. These reactions may occur as a consequence of expansion of the contents of the gastrointestinal tract, and an increase in motility due to the pharmacologic effects of PEG 3350. Mild diarrhoea usually responds to dose reduction.
Other adverse reactions which may occur are : Immune system disorders: allergic reactions, including anaphylaxis, angioedema, dyspnoea, rash, erythema, urticaria, and pruritis.
Metabolism and nutrition disorders: electrolyte disturbances, particularly hyperkalaemia and hypokalaemia.
Nervous system disorders: headache
Gastrointestinal disorders: abdominal pain, diarrhoea, vomiting, nausea, dyspepsia, abdominal distension, borborygmi, flatulence, and anal discomfort.
General disorders and administration site conditions: peripheral oedema.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting By reporting side effects, you can help provide more information on the safety of this medicine.
4.9. Overdose
Severe pain or distension, diarrhoea, vomiting may occur in case of overdosage.
Severe pain or distention can be treated by nasogastric aspiration.
Extensive fluid loss by diarrhoea or vomiting may require correction of electrolyte disturbances.
5.0 Pharmacological properties
5.1 Pharmacodynamic properties
Pharmacotherapeutic group : osmotically acting laxative.
Polyethylene glycol 3350 acts by virtue of its osmotic action in the gut, which induces a laxative effect. PEG 3350 increases the stool volume, triggering the colon motility via neuromuscular pathways leading to an improved propulsive colonic transportation of the softened stools and a facilitation of the defecation. Electrolytes combined with PEG 3350 are exchanged across the intestinal barrier (mucosa) with serum electrolytes and excreted in faecal water without net gain or loss of sodium, potassium and water.
5.2 Pharmacokinetic properties
PEG 3350 passes unchanged along the gut. It is virtually unabsorbed from the gastro- intestinal tract. Any polyethylene glycol 3350 that is absorbed is excreted via the urine.
6.0 Nonclinical properties
Preclinical studies provide evidence that macrogol or PEG 3350 has no significant systemic toxicity potential, based on conventional studies of pharmacology, repeated dose toxicity and genotoxicity.
There were no direct embryotoxic or teratogenic effects in rats even at maternally toxic levels that are a multiple of 66 x the maximum recommended dose in humans for chronic constipation and 25 x for faecal impaction. Indirect embryofetal effects, including reduction in fetal and placental weights, reduced fetal viability, increased limb and paw hyperflexion and abortions, were noted in the rabbit at a maternally toxic dose that was 3.3 x the maximum recommended dose in humans for treatment of chronic constipation and 1.3 x for faecal impaction. Rabbits are a sensitive animal test species to the effects of GI-acting substances and the studies were conducted under exaggerated conditions with high dose volumes administered, which are not clinically relevant. The findings may have been a consequence of an indirect effect of PEG 3350 related to poor maternal condition as the result of an exaggerated pharmacodynamic response in the rabbit. There was no indication of a teratogenic effect.
There are long-term animal toxicity and carcinogenicity studies involving macrogol 3350. Results from these and other toxicity studies using high levels of orally administered high molecular weight macrogols provide evidence of safety at the recommended therapeutic dose.
7.0 Description
Polyethylene glycols or Macrogols are addition polymers of ethylene oxide and water, represented by the chemical formula H(OCH CH ) OH, where n represents the average 2 2 n number of oxyethylene groups. Each macrogol is usually designated by a number that corresponds approximately to its average molecular weight. The chemical structure of PEG is given below:

n represents average no. of oxyethylene groups
Polyethylene glycol (PEG) 3350, a white or almost white solid with a waxy or paraffin like appearance has an approximate average molecular weight of 3350 daltons. Pegclear contains PEG 3350, Sodium Chloride, Sodium Bicarbonate and Potassium Chloride. When a 25 ml dose of Pegclear is made up to 125 ml of solution, it provides 65 mmol/l of Sodium, 53 mmol/l of Chloride, 5.4 mmol/I of Potassium & 17 mmol/l of Bicarbonate which corresponds to 8.125 mmol of Sodium, 6.625 mmol of Chloride, 0.675 mmol of Potassium and 2.125 mmol of Bicarbonate in each diluted dose of 125 ml.
8.0 Pharmaceutical particulars
8.1. Incompatibilities
Not applicable.
8.2. Shelf-life
24 months
8.3. Packaging information
A bottle of 200 ml
8.4. Storage and handing instructions
Store at a temperature not exceeding 30°C.
Do not refrigerate or freeze.
Keep out of reach of children.
Discard product 30 days after first opening. The diluted solution should be kept covered. Throw away any solution not used within a 24 hour period
9.0 Patient Counselling Information
Advise the patient to read the Patient Information Leaflet Instruct. patients:
- To reconstitute Pegclear oral solution with water prior to ingestion.
- Not to take other laxatives while they are taking Pegclear oral solution.
- Not to take oral medications within 1 hour before the start or during the administration of Pegclear oral solution.
- To take only clear liquids but avoid red and purple liquids.
- To consume water or other clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy.
- To follow the directions in the Instructions for Use on how to prepare and administer the product.
- If they experience severe bloating, distention or abdominal pain, to slow or temporarily discontinue drinking the solution and to contact their healthcare provider.
- To contact their healthcare provider if they develop signs and symptoms of dehydration or if they experience altered consciousness or seizures.
- To discontinue administration of the solution and contact their healthcare provider if they develop symptoms of a hypersensitivity reaction
12. Date of revision
12th June 2024
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you.
- Keep this leaflet. You may need to read it again.
- Ask your pharmacist if you need more information or advice.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
- You must talk to a doctor if you do not feel better or if you feel worse.
What is in this leaflet:
- What Pegclear is and what it is used for
- What you need to know before you take Pegclear
- How to take Pegclear
- Possible side effects
- How to store Pegclear
- Contents of the pack and other information
1. What PEGCLEARTM is and what it is used for
The name of this medicine is Pegclear concentrate for oral solution. It is a laxative for the treatment of chronic constipation in adults, adolescents and elderly and children. It is not recommended for children below 2 years of age.
Pegclear concentrate for oral solution helps you to have a comfortable bowel movement even if you have been constipated for a long time.
2. What you need to know before you take PEGCLEARTM
Do not take PEGCLEARTM if your doctor has told you that you have:
- a blockage in your intestine (gut obstruction, ileus)
- a perforated gut wall
- severe inflammatory bowel disease like ulcerative colitis, crohn’s disease or toxic megacolon
- an allergy to the active substances or any of the other ingredients of Pegclear concentrate for oral solution (section 6).
Warnings and precautions
When taking Pegclear you should continue to take plenty of fluids. The fluid content of Pegclear oral solution should not replace your regular liquid intake.
Pregnancy and breast-feeding
Pegclear can be taken during pregnancy and whilst breast feeding. If you are pregnant, trying to become pregnant or breast feeding, ask your pharmacist or doctor for advice before taking Pegclear.
Driving and using machines
Pegclear does not affect your ability to drive or use machines.
Do not exceed the stated dose.
3. How to take PEGCLEARTM
This medicine can be taken at any time with or without food.
Adults, adolescents & elderly:
This product must be diluted before use:
A dose of PEGCLEARTM concentrate for oral solution is 25 ml liquid diluted in 100 ml of water. Take this 1–3 times a day according to the severity of your constipation.
How to mix:
Open the bottle and measure 25 ml or five 5 ml teaspoonfuls. Pour the liquid into a glass and then add 100ml (about half a glassful) of water. Stir well until all the liquid has been evenly mixed and the diluted Pegclear concentrate for oral solution is clear, then drink it. Please rinse the dosing cup after use and replace on the bottle.
Children:
2 to 6 years: usual starting daily dose is 12.5 ml solution diluted in 50 ml of water.
7 to 11 years: usual starting daily dose is 25 ml solution diluted in 100 ml of water.
The dose should be adjusted up or down as required to produce regular soft stools. If the dose needs increasing, this is best done every second day.
The maximum dose of PEG 3350 does not normally exceed 50 ml of PEGCLEARTM concentrate for oral solution per day.
Geriatric use: Can be administered to elderly patients in the same dosage as recommended for adults
Duration of treatment:
Treatment with Pegclear concentrate for oral solution usually lasts for about 2 weeks. If you need to take Pegclear concentrate for oral solution for longer, please see your doctor. If your constipation is caused by an illness such as Parkinson’s disease or multiple sclerosis (MS), or if you take medicines that cause constipation your doctor may recommend that you take Pegclear concentrate for oral solution for longer than 2 weeks. Usually for long term treatment the dose can be lowered to either 1 or 2 doses a day. If you take more Pegclear concentrate for oral solution than you should You may develop excessive diarrhoea, which can lead to dehydration. If this occurs, stop taking Pegclear concentrate for oral solution and drink plenty of fluids. If you are worried contact your doctor or pharmacist.
If you forget to take PEGCLEARTM
Take the dose as soon as you remember to take it.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you have a serious allergic reaction which causes difficulty in breathing, or swelling of the face, lips, tongue or throat, tell your doctor immediately and stop taking Pegclear. Other allergic reactions may occur which may cause a skin rash,itching, reddening of the skin or a nettle rash, swollen hands, feet or ankles and headache.
Other side effects may also include indigestion, stomach ache and rumbles and high and low levels of potassium in the blood. You may also feel bloated, suffer from wind, feel sick or vomit, may also experience soreness of the anus (bottom) and may have mild diahorrea when starting to take Pegclear which generally gets better if you reduce the amount of Pegclear you take.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store PEGCLEARTM
Keep this medicine out of the sight and reach of children.
Do not use PEGCLEARTM after the expiry date which is stated on the bottle. The expiry date refers to the last day of that month.
Do not refrigerate or freeze. Store at a temperature not exceeding 30°C.
Discard any remaining product 30 days after first opening the bottle. Once you have diluted Pegclear in water, if you cannot drink it straight away keep it covered. Throw away any solution not used within a 24 hour period. Do not throw away any medicines via wastewater or household waste.
Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What PEGCLEARTM contains
Each 25 ml of Pegclear concentrate for oral solution contains the following:
Polyethylene Glycol 3350 USP 13.125 gm
Sodium Chloride IP 0.3507 gm
Sodium Bicarbonate IP 0.1785 gm
Potassium Chloride IP 0.0466 gm
What PEGCLEARTM looks like and contents of the pack
Pegclear concentrate for oral solution is a clear, colourless liquid.
Each pack consists of a carton with a 200 ml bottle of solution and a plastic dosing cup.
Marketing Authorisation Holder
Zuventus Healthcare Ltd Survey No. 6/2, Aladahalli Village, Sompura Hobli,
Nelamangala Taluk, Bengaluru Rural - 562 111, India.
TM - Trade Mark Owners.
This leaflet was last revised in 12/06/2024