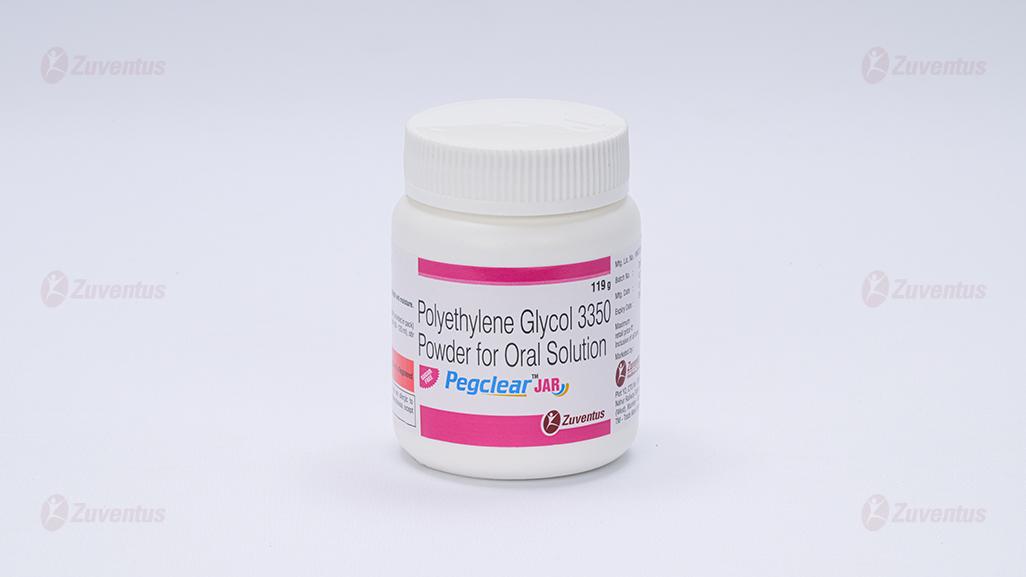
Pegclear Jar
Therapy Area
Gastrointestinal
1.0 Generic Name
Polyethylene Glycol 3350 Powder for Oral Solution
2.0 Qualitative and quantitative composition
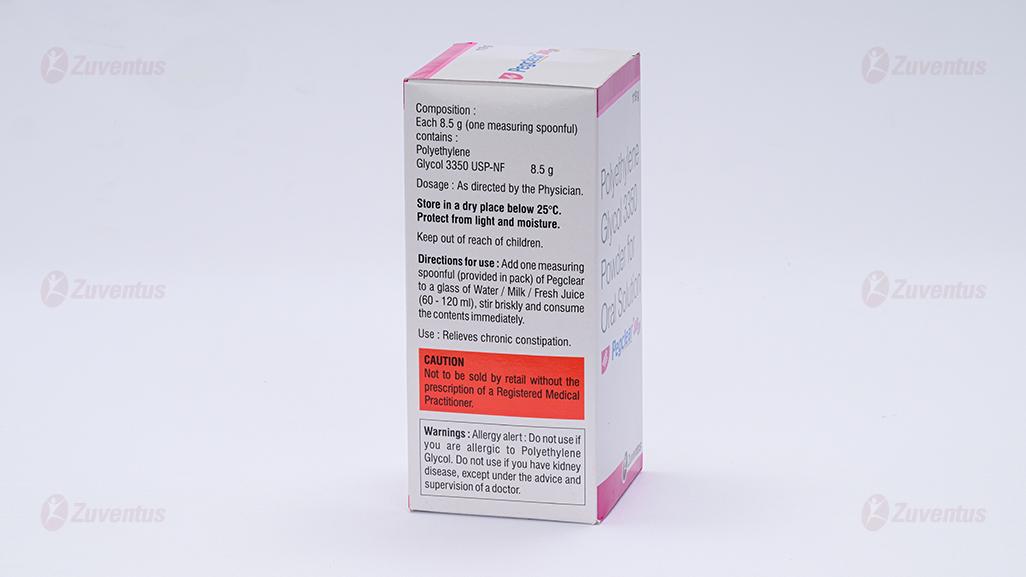
Composition:
Each 8.5 g (one measuring spoonful) contains:
Polyethylene Glycol 3350 USP-NF……………8.5 g
3.0 Dosage form and strength
Dosage Form: Powder for Oral Solution
Dosage Strength: 8.5 g per one measuring spoonful
4.0 Clinical particulars
4.1 Therapeutic indication
For the treatment of occasional constipation.
This product should be used for 2 weeks or less or as directed by a physician.
4.2 Posology and method of administration
Dosage:
Adults: The usual dose is 17 grams (2 scoops) of powder per day. Take 2 scoops of powder & add water to make it to the mark of 125 ml/dose. It can be taken 1 to 3 times per day according to individual response.
Pediatrics:
Children 2 - 6 years - Take 1 scoop of Powder & add water to make it to the mark of 62.5 ml/dose.
Children 6 years & above - Take 2 scoops of Powder & add water to make it to the mark of 125 ml/dose. Dose should be adjusted up or down as required to produce regular soft stools. The maximum dose does not normally exceed 3 scoops per day for both pediatric age groups.
Directions for use:
Each PeclearTM Jar is supplied with a measuring spoon to contain 8.5 grams of laxative powder when filled. Water/milk/fresh Juice can be used to dissolve the powder. Stir briskly and consume the contents immediately. 1 to 3 days may be required to produce a bowel movement.
4.3 Contraindications
Pegclear Powder for Oral Solution is contraindicated in patients with known or suspected bowel obstruction and patients known to be allergic to polyethylene glycol.
4.4 Special warnings and precautions for use
Warnings
Patients with symptoms suggestive of bowel obstruction (nausea, vomiting, abdominal pain or distention) should be evaluated to rule out this condition before initiating PegclearTM therapy.
Precautions
General: Patients presenting with complaints of constipation should have a thorough medical history and physical examination to detect associated metabolic, endocrine and neurogenic conditions, and medications. A diagnostic evaluation should include a structural examination of the colon. Patients should be educated about good defecatory and eating habits (such as high fiber diets) and lifestyle changes (adequate dietary fiber and fluid Intake, regular exercise) which may produce more regular bowel habits.
Laboratory Tests: No clinically significant effect on laboratory tests have been demonstrated.
4.5 Drug interactions
No specific drug Interactions have been demonstrated.
4.6 Use in special populations
Pregnancy:
There are limited amount of data from the use of PegclearTM in pregnant women. Studies in animals have shown indirect reproductive toxicity. Clinically, no effects during pregnancy are anticipated, since systemic exposure to polyethylene glycol 3350 is negligible. PegclearTM can be used during pregnancy.
Lactation:
As systemic exposure of the breast-feeding woman to PEG 3350 is negligible, no effects on the breastfed newborn/infant are anticipated, and PEG 3350 can be used during breast-feeding.
Geriatric Use:
There is no evidence for special considerations when PegclearTM is administered to elderly patients. In geriatric nursing home patients, a higher incidence of diarrhea occurred at the recommended 17 g dose. If diarrhea occurs PegclearTM should be discontinued.
4.7 Effects on ability to drive and use machines
Polyethylene Glycol 3350 Powder for Oral Solution has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Nausea, abdominal bloating, cramping and flatulence may occur. High doses may produce diarrhea and excessive stool frequency, particularly in elderly nursing home patients.
Patients taking other medications containing polyethylene glycol have occasionally developed urticaria suggestive of an allergic reaction.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
There have been no reports of accidental overdosage. In the event of overdosage diarrhea would be the expected major event. Severe pain or distension, diarrhoea, vomiting may occur in case of overdosage. Medication should be terminated and free water administered. Severe pain or distention can be treated by nasogastric aspiration. Extensive fluid loss by diarrhoea or vomiting may require correction of electrolyte disturbances.
The oral LD50 Is >50 gm/kg in mice, rats and rabbits.
5.0 Pharmacological properties
5.1 Mechanism of Action
Pharmacotherapeutic group: osmotically acting laxative.
Polyethylene glycol 3350 acts by virtue of its osmotic action in the gut, which induces a laxative effect. PEG 3350 increases the stool volume, triggering the colon motility via neuromuscular pathways leading to an improved propulsive colonic transportation of the softened stools and a facilitation of the defecation.
5.2 Pharmacodynamic properties
Pharmacodynamic effect of PEG 3350 as a laxative is primarily due to its osmotic action, which increases water content in the stool, softens it, and facilitates easier passage of stool, thereby relieving constipation. In clinical studies an increase in frequency bowel movement and daily stool weight was observed.
In vitro study showed indirectly that PEG 3350 was not fermented into hydrogen or methane by the colonic microflora in human feces. PEG 3350 appears to have no effect on the active absorption or secretion of glucose or electrolytes. There is no evidence of tachyphylaxis.
6.0 Nonclinical properties
6.1 Animal Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long term carcinogenicity studies, genetic toxicity studies and reproductive toxicity studies in animal have not been performed with PegclearTM.
7.0 Description
PEG 3350 is an osmotic agent for the treatment of constipation.
PegclearTM (polyethylene glycol 3350, NF) is a synthetic polyglycol having an average molecular weight of 3950.
The chemical formula is HO(C₂H₂O)nH in which n represents the average number of oxyethylene groups.
Below 55°C it is a free flowing white powder freely soluble in water.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None applicable
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
PegclearTM Jar contains 119 g of PEG 3350 Powder for Oral Solution
8.4 Storage and handing instructions
Store in a dry place below 25°C.
Protect from light and moisture.
Keep out of reach of children.
9.0 Patient Counselling Information
- PEG 3350 softens the stool and Increases the frequency of bowel movements by retaining water in the stool.
- It should always be taken by mouth after being dissolved in Water/milk/fresh Juice. Should unusual cramps, bloating, or diarrhea occur, consult your physician.
- 1 to 3 days may be required to produce a bowel movement.
- This product should be used for 2 weeks or less or as directed by a physician.
- Prolonged, frequent or excessive use of PEG 3350 may result in electrolyte Imbalance and dependence on laxatives.
12.0 Date of revision
28th June 2024
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse has told you.
- Keep this leaflet. You may need to read it again.
- Ask your pharmacist if you need more information or advice.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
- You must talk to a doctor if you do not feel better or if you feel worse.
What is in this leaflet:
1. What PEGCLEARTM is and what it is used for
2. What you need to know before you take PEGCLEARTM
3. How to take PEGCLEARTM
4. Possible side effects
5. How to store PEGCLEARTM
6. Contents of the pack and other information
1. What PEGCLEAR is and what it is used for
The name of this medicine is Polyethylene Glycol 3350 Powder for Oral Solution. It is a laxative for the treatment of chronic constipation in adults, adolescents and elderly and children. It is not recommended for children below 2 years of age.
Polyethylene Glycol 3350 Powder for Oral Solution helps you to have a comfortable bowel movement even if you have been constipated for a long time.
2. What you need to know before you take PEGCLEAR
Do not take PEGCLEARTM if your doctor has told you that you have:
- a blockage in your intestine (gut obstruction, ileus)
- a perforated gut wall
- severe inflammatory bowel disease like ulcerative colitis, crohn’s disease or toxic megacolon
- an allergy to the active substances or any of the other ingredients of PegclearTM.
Warnings and precautions
When taking PEGCLEARTM you should continue to take plenty of fluids. The fluid content of Pegclear oral solution should not replace your regular liquid intake.
Pregnancy and breast-feeding
PEGCLEARTM can be taken during pregnancy and whilst breast feeding. If you are pregnant, trying to become pregnant or breast feeding, ask your pharmacist or doctor for advice before taking PEGCLEARTM.
Driving and using machines
PEGCLEARTM does not affect your ability to drive or use machines.
Do not exceed the stated dose.
3. How to take PEGCLEAR
This medicine can be taken at any time with or without food.
Adults
The usual dose is 17 grams (2 scoops) of powder per day. Take 2 scoops of powder & add water to make it to the mark of 125 ml/dose. It can be taken 1 to 3 times per day according to individual response.
Pediatrics:
Children 2 - 6 years - Take 1 scoop of Powder & add water to make it to the mark of 62.5 ml/dose.
Children 6 years & above - Take 2 scoops of Powder & add water to make it to the mark of 125 ml/dose. Dose should be adjusted up or down as required to produce regular soft stools. The maximum dose does not normally exceed 3 scoops per day for both pediatric age groups.
Directions for use:
Each PeclearTM Jar is supplied with a measuring spoon to contain 8.5 grams of laxative powder when filled. Water/milk/fresh Juice can be used to dissolve the powder. Stir briskly and consume the contents immediately. 1 to 3 days may be required to produce a bowel movement.
Geriatric use: Can be administered to elderly patients in the same dosage as recommended for adults
Duration of treatment: Treatment with PeclearTM usually lasts for about 2 weeks. If you need to take PeclearTM for longer, please see your doctor. If your constipation is caused by an illness such as Parkinson’s disease or multiple sclerosis (MS), or if you take medicines that cause constipation your doctor may recommend that you take PeclearTM for longer than 2 weeks. Usually for long term treatment the dose can be lowered to either 1 or 2 doses a day. If you take more PeclearTM than you should, you may develop excessive diarrhoea, which can lead to dehydration. If this occurs, stop taking PegclearTM and drink plenty of fluids. If you are worried contact your doctor or pharmacist.
If you forget to take PEGCLEARTM
Take the dose as soon as you remember to take it.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you have a serious allergic reaction which causes difficulty in breathing, or swelling of the face, lips, tongue or throat, tell your doctor immediately and stop taking Pegclear.
Other allergic reactions may occur which may cause a skin rash, itching, reddening of the skin or a nettle rash, swollen hands, feet or ankles and headache.
Other side effects may also include indigestion, stomach ache and rumbles and high and low levels of potassium in the blood. You may also feel bloated, suffer from wind, feel sick or vomit, may also experience soreness of the anus (bottom) and may have mild diahorrea when starting to take Pegclear which generally gets better if you reduce the amount of Pegclear you take.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store PEGCLEAR
Keep this medicine out of the sight and reach of children.
Do not use PeclearTM Jar after the expiry date which is stated on the Jar. The expiry date refers to the last day of that month.
Do not refrigerate or freeze. Store at a temperature not exceeding 25°C.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What PEGCLEARTM contains
Each 8.5 g (one measuring spoonful) contains:
Polyethylene Glycol 3350 USP-NF……………8.5 g
What PEGCLEARTM looks like and contents of the pack
PegclearTM Jar contains 119 g of PEG 3350 Powder for Oral Solution with calibrated 125 ml measuring cup.
Marketing Authorisation Holder
Zuventus Healthcare Limited
Plot Y2, CTS No : 358/A2, Near
Nahur Railway Station, Nahur
(West), Mumbai - 400 078, India.
TM - Trade Mark Owners.
This leaflet was last revised in 01/07/2024