Rabifast-40 Tablets
Therapy Area
Gastrointestinal
1.0 Name of the medicinal product
Rabeprazole Gastro-resistant Tablets IP
2.0 Qualitative and quantitative composition
Rabifast 20
Each gastro-resistant tablet contains:
Rabiprazole Sodium IP 20 mg
Excipients q.s.
Colours: Yellow Oxide of Iron & Titanium Dioxide IP
Rabifast 40
Each gastro-resistant tablet contains:
Rabiprazole Sodium IP 40 mg
Excipients q.s.
Colours: Ferric oxide Yellow USPNF & Titanium Dioxide IP
3.0 Dosage form and strength
Tablet
20 mg and 40 mg
4.0 Clinical Particulars
4.1 Therapeutic indication
For the treatment of gastroesophageal reflux disease, duodenal ulcer & zollinger ellision syndrome.
4.2 Posology and method of administration
Adults /older people
Active Duodenal Ulcer and Active Benign Gastric Ulcer: The recommended oral dose for both active duodenal ulcer and active benign gastric ulcer is 20 mg to be taken once daily in the morning. Most patients with active duodenal ulcer heal within four weeks. However, a few patients may require an additional four weeks of therapy to achieve healing. Most patients with active benign gastric ulcer heal within six weeks. However again a few patients may require an additional six weeks of therapy to achieve healing.
Erosive or Ulcerative Gastroesophageal Reflux Disease (GERD): The recommended oral dose for this condition is 20 mg to be taken once daily for four to eight weeks.
Gastroesophageal Reflux Disease Long-term Management (GERD Maintenance): For long-term management, a maintenance dose of Rabifast 20 mg once daily can be used depending upon patient response.
Symptomatic treatment of moderate to very severe gastroesophageal reflux disease (symptomatic GERD): 20 mg once daily is recommended for the treatment of daytime and nighttime heartburn and other symptoms associated with GERD in adults for up to 4 weeks.
Zollinger-Ellison Syndrome: The recommended adult starting dose is 60 mg once a day. The dose may be titrated upwards to 120 mg/day based on individual patient needs. Single daily doses up to 100 mg/day may be given. 120 mg dose may require divided doses, 60 mg twice daily. Treatment should continue for as long as clinically indicated.
For indications requiring once-daily treatment Rabifast tablets should be taken in the morning, before eating; and although neither the time of day nor food intake was shown to have any effect on rabeprazole sodium activity, this regimen will facilitate treatment compliance.
Renal and hepatic impairment
No dosage adjustment is necessary for patients with renal or hepatic impairment.
See section 4.4 in the treatment of patients with severe hepatic impairment.
Children
Rabifast is not recommended for use in children, as there is no experience of its use in this group.
Method of administration
Patients should be cautioned that the Rabifast tablets should not be chewed or crushed, but should be swallowed whole.
4.3 Contraindications
- Hypersensitivity to the active substance or to any of the excipients.
- Rabifast is contraindicated in pregnancy and during breast-feeding (see section 4.6).
4.4 Special warnings and precautions for use
- Symptomatic response to therapy with rabeprazole sodium does not preclude the presence of gastric or oesophageal malignancy, therefore the possibility of malignancy should be excluded prior to commencing treatment with Rabifast tablets.
- Patients on long-term treatment (particularly those treated for more than a year) should be kept under regular surveillance.
- A risk of cross-hypersensitivity reactions with other proton pump inhibitor (PPI) or substituted benzimidazoles cannot be excluded.
- Rabifast tablets are not recommended for use in children, as there is no experience of its use in this group.
- There have been post marketing reports of blood dyscrasias (thrombocytopenia and neutropenia). In the majority of cases where an alternative aetiology cannot be identified, the events were uncomplicated and resolved on discontinuation of rabeprazole.
- Hepatic enzyme abnormalities have been seen in clinical trials and have also been reported since market authorisation. In the majority of cases where an alternative aetiology cannot be identified, the events were uncomplicated and resolved on discontinuation of rabeprazole.
- No evidence of significant drug related safety problems was seen in a study of patients with mild to moderate hepatic impairment versus normal age and sex matched controls. However, because there are no clinical data on the use of Rabifast in the treatment of patients with severe hepatic dysfunction the prescriber is advised to exercise caution when treatment with Rabifast is first initiated in such patients.
- Co-administration of atazanavir with Rabifast is not recommended (see section 4.5).
- Treatment with PPIs, including Rabifast, may possibly increase the risk of gastrointestinal infections such as Salmonella, Campylobacter and Clostridium difficile (see section 5.2).
- PPIs, especially if used in high doses and over long durations (>1 year), may modestly increase the risk of hip, wrist and spine fracture, predominantly in older people or in presence of other recognised risk factors. Observational studies suggest that PPIs may increase the overall risk of fracture by 10– 40%. Some of this increase may be due to other risk factors. Patients at risk of osteoporosis should receive care according to current clinical guidelines and they should have an adequate intake of vitamin D and calcium.
- Severe hypomagnesaemia has been reported in patients treated with PPIs like Rabifast for at least three months, and in most cases for a year. Serious manifestations of hypomagnesaemia such as fatigue, tetany, delirium, convulsions, dizziness and ventricular arrhythmia can occur but they may begin insidiously and be overlooked. In most affected patients, hypomagnesaemia improved after magnesium replacement and discontinuation of the PPI.
- For patients expected to be on prolonged treatment or who take PPIs with digoxin or drugs that may cause hypomagnesaemia (e.g., diuretics), health care professionals should consider measuring magnesium levels before starting PPI treatment and periodically during treatment.
Concomitant use of rabeprazole with methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients.
Influence on vitamin B12 absorption
Rabeprazole sodium, as all acid-blocking medicines, may reduce the absorption of vitamin B12 (cyanocobalamin) due to hypo- or a- chlorhydria. This should be considered in patients with reduced body stores or risk factors for reduced vitamin B12 absorption on long-term therapy or if respective clinical symptoms are observed.
Subacute cutaneous lupus erythematosus (SCLE)
PPIs are associated with very infrequent cases of SCLE. If lesions occur, especially in sun-exposed areas of the skin, and if accompanied by arthralgia, the patient should seek medical help promptly and the health care professional should consider stopping Rabifast. SCLE after previous treatment with a PPI may increase the risk of SCLE with other PPIs.
Interference with laboratory tests
Increased Chromogranin A (CgA) level may interfere with investigations for neuroendocrine tumours. To avoid this interference, Rabifast treatment should be stopped for at least 5 days before CgA measurements. If CgA and gastrin levels have not returned to reference range after initial measurement, measurements should be repeated 14 days after cessation of PPI treatment.
Renal impairment
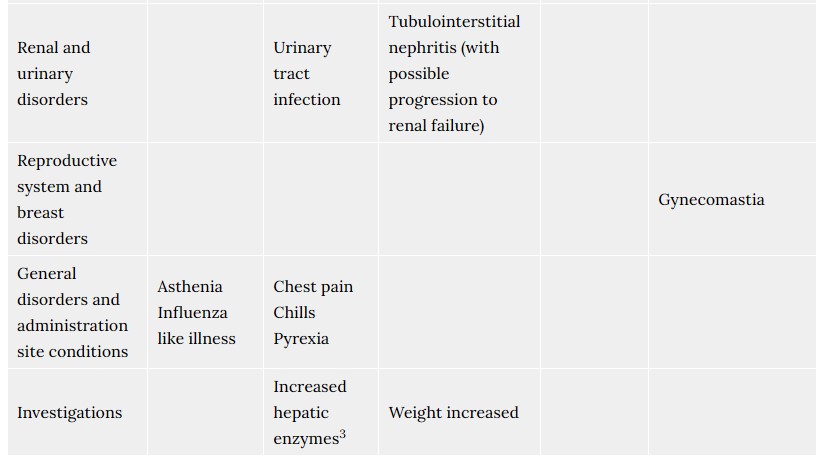
Acute tubulointerstitial nephritis (TIN) has been observed in patients taking rabeprazole and may occur at any point during rabeprazole therapy (see section 4.8). Acute tubulointerstitial nephritis can progress to renal failure.
Rabeprazole should be discontinued in case of suspected TIN, and appropriate treatment should be promptly initiated
4.5 Drug interactions
Rabeprazole sodium produces a profound and long lasting inhibition of gastric acid secretion. An interaction with compounds whose absorption is pH dependent may occur. Co-administration of rabeprazole sodium with ketoconazole or itraconazole may result in a significant decrease in antifungal plasma levels. Therefore, individual patients may need to be monitored to determine if a dosage adjustment is necessary when ketoconazole or itraconazole are taken concomitantly with Rabifast Tablets.
In clinical trials, antacids were used concomitantly with the administration of Rabifast Tablets and, in a specific drug-drug interaction study, no interaction with liquid antacids was observed.
Co-administration of atazanavir 300 mg/ritonavir 100 mg with omeprazole (40 mg once daily) or atazanavir 400 mg with lansoprazole (60 mg once daily) to healthy volunteers resulted in a substantial reduction in atazanavir exposure. The absorption of atazanavir is pH dependent. Although not studied, similar results are expected with other PPIs. Therefore, PPIs, including rabeprazole, should not be co-administered with atazanavir (see section 4.4).
Methotrexate
Case reports, published population pharmacokinetic studies, and retrospective analyses suggest that concomitant administration of PPIs and methotrexate (primarily at high dose) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate. However, no formal drug interaction studies of methotrexate with PPIs have been conducted.
4.6 Use in special populations
Pregnancy
There are no data on the safety of rabeprazole in human pregnancy.
Reproduction studies performed in rats and rabbits have revealed no evidence of impaired fertility or harm to the foetus due to rabeprazole sodium, although low foeto-placental transfer occurs in rats. Rabifast Tablets is contraindicated during pregnancy.
Breast-feeding
It is not known whether rabeprazole sodium is excreted in human breast milk. No studies in breast feeding women have been performed. Rabeprazole sodium is however excreted in rat mammary secretions. Therefore, Rabifast Tablets should not be used during breast feeding.
4.7 Effects on the ability to drive and use machines
Based on the pharmacodynamic properties and the adverse events profile, it is unlikely that Rabifast Tablets would cause an impairment of driving performance or compromise the ability to use machinery. If however, alertness is impaired due to somnolence, it is recommended that driving and operating complex machinery be avoided.
4.8 Undesirable effects
The most commonly reported adverse drug reactions, during controlled clinical trials with rabeprazole were headache, diarrhoea, abdominal pain, asthenia, flatulence, rash and dry mouth. The majority of adverse events experienced during clinical studies were mild or moderate in severity, and transient in nature. The following adverse events have been reported from clinical trial and post-marketing experience.
Frequencies are defined as: common (> 1/100, < 1/10), uncommon (> 1/1,000, < 1/100), rare (>1/10,000, <1/1000) very rare (<1/10,000), not known (cannot be estimated from the available data).
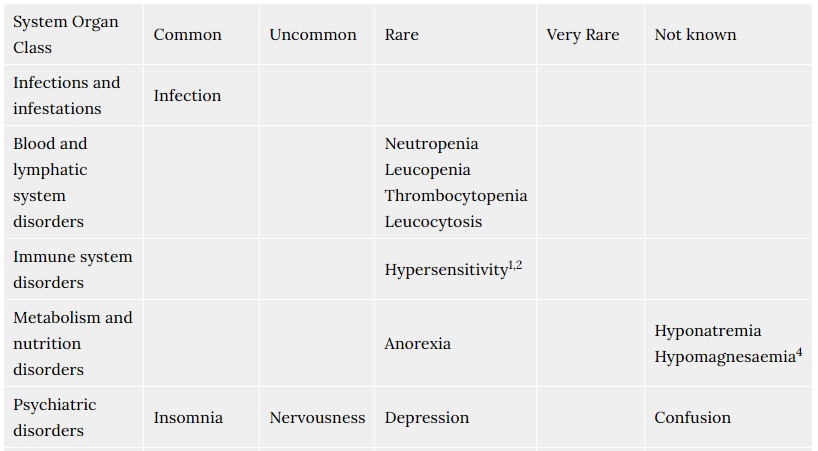
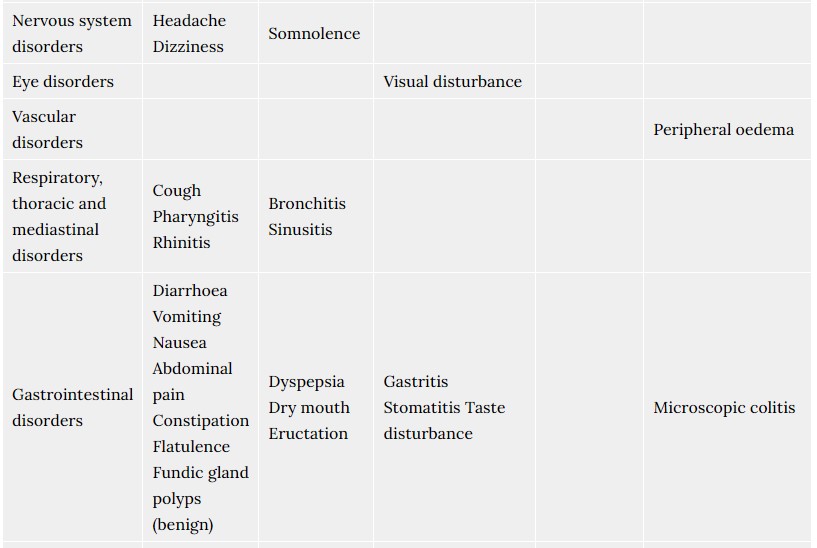
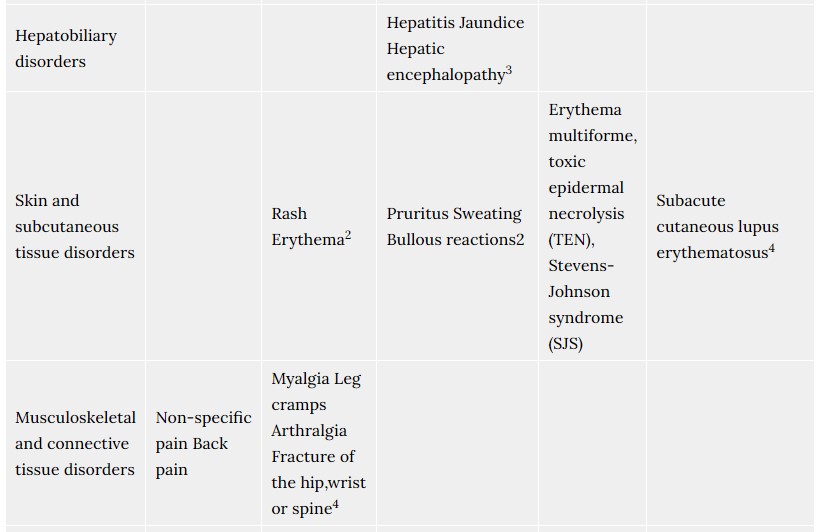
- Includes facial swelling, hypotension and dyspnoea
- Erythema, bullous reactions and hypersensitivity reactions have usually resolved after discontinuation of therapy.
- Rare reports of hepatic encephalopathy have been received in patients with underlying cirrhosis. In treatment of patients with severe hepatic dysfunction the prescriber is advised to exercise caution when treatment with Rabifast Tablets are first initiated in such patients (see section 4.4).
- See Special warnings and precautions for use.
Reporting of side effects
Reporting suspected adverse reactions after authorisation of the medicinal product is important.
It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Experience to date with deliberate or accidental overdose is limited. The maximum established exposure has not exceeded 60 mg twice daily, or 160 mg once daily. Effects are generally minimal, representative of the known adverse event profile and reversible without further medical intervention. No specific antidote is known. Rabeprazole sodium is extensively protein bound and is, therefore, not dialysable. As in any case of overdose, treatment should be symptomatic and general supportive measures should be utilised.
5.0 Pharmacological Properties
5.1 Mechanism of Action
Pharmaco-therapeutic group: Alimentary tract and metabolism, Drugs for peptic ulcer and gastro-esophageal reflux disease (GERD), PPIs,
ATC code: A02B C04
Rabeprazole sodium belongs to the class of anti-secretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or H2 histamine antagonist properties, but suppress gastric acid secretion by the specific inhibition of the H+/K+-ATPase enzyme (the acid or proton pump) The effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus. Animal studies indicate that after administration, rabeprazole sodium rapidly disappears from both the plasma and gastric mucosa. As a weak base, rabeprazole is rapidly absorbed following all doses and is concentrated in the acid environment of the parietal cells. Rabeprazole is converted to the active sulphenamide form through protonation and it subsequently reacts with the available cysteines on the proton pump.
5.2 Pharmacodynamic properties
Anti-secretory activity
After oral administration of a 20 mg dose of rabeprazole sodium the onset of the anti-secretory effect occurs within one hour, with the maximum effect occurring within two to four hours. Inhibition of basal and food stimulated acid secretion 23 hours after the first dose of rabeprazole sodium are 69% and 82% respectively and the duration of inhibition lasts up to 48 hours. The inhibitory effect of rabeprazole sodium on acid secretion increases slightly with repeated once-daily dosing, achieving steady state inhibition after three days. When the drug is discontinued, secretory activity normalises over 2 to 3 days.
Decreased gastric acidity due to any means, including PPIs such as rabeprazole, increases counts of bacteria normally present in the gastrointestinal tract. Treatment with PPIs may possibly increase the risk of gastrointestinal infections such as Salmonella, Campylobacter and Clostridium difficile.
Serum gastrin effects
In clinical studies patients were treated once daily with 10 or 20 mg rabeprazole sodium, for up to 43 months duration. Serum gastrin levels increased during the first 2 to 8 weeks reflecting the inhibitory effects on acid secretion and remained stable while treatment was continued. Gastrin values returned to pre-treatment levels, usually within 1 to 2 weeks after discontinuation of therapy.
Human gastric biopsy specimens from the antrum and the fundus from over 500 patients receiving rabeprazole or comparator treatment for up to 8 weeks have not detected changes in ECL cell histology, degree of gastritis, incidence of atrophic gastritis, intestinal metaplasia or distribution of H. pylori infection. In over 250 patients followed for 36 months of continuous therapy, no significant change in findings present at baseline was observed.
Other effects
Systemic effects of rabeprazole sodium in the CNS, cardiovascular and respiratory systems have not been found to date. Rabeprazole sodium, given in oral doses of 20 mg for 2 weeks, had no effect on thyroid function, carbohydrate metabolism, or circulating levels of parathyroid hormone, cortisol, oestrogen, testosterone, prolactin, cholecystokinin, secretin, glucagon, follicle stimulating hormone (FSH), luteinising hormone (LH), renin, aldosterone or somatotrophic hormone.
Studies in healthy subjects have shown that rabeprazole sodium does not have clinically significant interactions with amoxicillin. Rabeprazole does not adversely influence plasma concentrations of amoxicillin or clarithromycin when co-administered for the purpose of eradicating upper gastrointestinal H. pylori infection.
During treatment with antisecretory medicinal products, serum gastrin increases in response to the decreased acid secretion. Also CgA increases due to decreased gastric acidity. The increased CgA level may interfere with investigations for neuroendocrine tumours.
Available published evidence suggests that PPIs should be discontinued between 5 days and 2 weeks prior to CgA measurements. This is to allow CgA levels that might be spuriously elevated following PPI treatment to return to reference range.
5.3 Pharmacokinetic properties
Absorption
Rabifast Tablet is an gastro-resistant tablet formulation of rabeprazole sodium. This presentation is necessary because rabeprazole is acid-labile. Absorption of rabeprazole therefore begins only after the tablet leaves the stomach. Absorption is rapid, with peak plasma levels of rabeprazole occurring approximately 3.5 hours after a 20 mg dose. Peak plasma concentrations (Cmax) of rabeprazole and AUC are linear over the dose range of 10 mg to 40 mg. Absolute bioavailability of an oral 20 mg dose (compared to intravenous administration) is about 52% due in large part to pre-systemic metabolism. Additionally, the bioavailability does not appear to increase with repeat administration. In healthy subjects the plasma half-life is approximately one hour (range 0.7 to 1.5 hours), and the total body clearance is estimated to be 283 ± 98 ml/min. There was no clinically relevant interaction with food. Neither food nor the time of day of administration of the treatment affect the absorption of rabeprazole sodium.
Distribution
Rabeprazole is approximately 97% bound to human plasma proteins.
Metabolism and excretion
Rabeprazole sodium, as is the case with other members of the PPI class of compounds, is metabolised through the cytochrome P450 (CYP450) hepatic drug metabolising system. In vitro studies with human liver microsomes indicated that rabeprazole sodium is metabolised by isoenzymes of CYP450 (CYP2C19 and CYP3A4). In these studies, at expected human plasma concentrations rabeprazole neither induces nor inhibits CYP3A4; and although in vitro studies may not always be predictive of in vivo status these findings indicate that no interaction is expected between rabeprazole and cyclosporin. In humans the thioether (M1) and carboxylic acid (M6) are the main plasma metabolites with the sulphone (M2), desmethyl-thioether (M4) and mercapturic acid conjugate (M5) minor metabolites observed at lower levels. Only the desmethyl metabolite (M3) has a small amount of anti-secretory activity, but it is not present in plasma.
Following a single 20 mg 14C labelled oral dose of rabeprazole sodium, no unchanged drug was excreted in the urine. Approximately 90% of the dose was eliminated in urine mainly as the two metabolites: a mercapturic acid conjugate (M5) and a carboxylic acid (M6), plus two unknown metabolites. The remainder of the dose was recovered in faeces.
Gender
Adjusted for body mass and height, there are no significant gender differences in pharmacokinetic parameters following a single 20 mg dose of rabeprazole.
Renal dysfunction
In patients with stable, end-stage, renal failure requiring maintenance haemodialysis (creatinine clearance ≤ 5ml/min/1.73 m2), the disposition of rabeprazole was very similar to that in healthy volunteers. The AUC and the Cmax in these patients was about 35% lower than the corresponding parameters in healthy volunteers. The mean half-life of rabeprazole was 0.82 hours in healthy volunteers, 0.95 hours in patients during haemodialysis and 3.6 hours post dialysis. The clearance of the drug in patients with renal disease requiring maintenance haemodialysis was approximately twice that in healthy volunteers.
Hepatic dysfunction
Following a single 20 mg dose of rabeprazole to patients with chronic mild to moderate hepatic impairment the AUC doubled and there was a 2-3 fold increase in half-life of rabeprazole compared to the healthy volunteers. However, following a 20 mg dose daily for 7 days the AUC had increased to only 1.5-fold and the Cmax to only 1.2-fold. The half-life of rabeprazole in patients with hepatic impairment was 12.3 hours compared to 2.1 hours in healthy volunteers. The pharmacodynamic response (gastric pH control) in the two groups was clinically comparable.
Older people
Elimination of rabeprazole was somewhat decreased in older people. Following 7 days of daily dosing with 20 mg of rabeprazole sodium, the AUC approximately doubled, the Cmax increased by 60% and t½ increased by approximately 30% as compared to young healthy volunteers. However, there was no evidence of rabeprazole accumulation.
CYP2C19 polymorphism
Following a 20 mg daily dose of rabeprazole for 7 days, CYP2C19 slow metabolisers, had AUC and t½ which were approximately 1.9 and 1.6 times the corresponding parameters in extensive metabolisers whilst Cmax had increased by only 40%.
6.0 Nonclinical Properties
6.1 Animal Toxicology or Pharmacology
Non-clinical effects were observed only at exposures sufficiently in excess of the maximum human exposure that make concerns for human safety negligible in respect of animal data.
Studies on mutagenicity gave equivocal results. Tests in mouse lymphoma cell line were positive, but in vivo micronucleus and in vivo and in vitro DNA repair tests were negative. Carcinogenicity studies revealed no special hazard for humans.
7.0 Description
The active ingredient in Rabifast Tablets is rabeprazole sodium, a substituted benzimidazole that inhibits gastric acid secretion. Rabeprazole sodium is a white to slightly yellowish-white solid. It is very soluble in water and methanol, freely soluble in ethanol, chloroform and ethyl acetate and insoluble in ether and n-hexane. The stability of rabeprazole sodium is a function of pH; it is rapidly degraded in acid media, and is more stable under alkaline conditions. IUPAC Name: Sodium;2-[[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl]-benzimidazol-1-ide
Molecular Formula: C18H20N3NaO3S
Molecular Weight: 381.43 g/mol
Structure:

8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf life
Refer on the pack.
8.3 Packaging Information
Rabifast 20 Tablets: 10 Blister strips of 15 tablets each
Rabifast 40 Tablets: 10 Blister strips of 10 tablets each
8.4 Storage and handling instructions
Store below 30ºC. Protect from light and moisture.
Keep out of reach of children.
Tablets should be swallowed whole & not to be broken, chewed, or crushed.
9.0 Patient Counselling Information
- Acute Interstitial Nephritis
Advise the patient or caregiver to call the patient’s healthcare provider immediately if they experience signs and/or symptoms associated with acute interstitial nephritis
- Clostridium difficile-Associated Diarrhea
Advise the patient or caregiver to immediately call the patient’s healthcare provider if they experience diarrhea that does not improve
- Bone Fracture
Advise the patient or caregiver to report any fractures, especially of the hip, wrist or spine, to the patient’s healthcare provider
- Severe Cutaneous Adverse Reactions
Advise the patient or caregiver to discontinue Rabifast Tablets and report any signs and symptoms of a severe cutaneous adverse reaction or other sign of hypersensitivity to the healthcare provider
- Cutaneous and Systemic Lupus Erythematosus
Advise the patient or caregiver to immediately call the patient’s healthcare provider for any new or worsening of symptoms associated with cutaneous or systemic lupus erythematosus.
- Cyanocobalamin (Vitamin B-12) Deficiency
Advise the patient or caregiver to report any clinical symptoms that may be associated with cyanocobalamin deficiency to the patient’s healthcare provider if they have been receiving rabeprazole sodium tablets for longer than 3 years
- Hypomagnesemia
Advise the patient or caregiver to report any clinical symptoms that may be associated with hypomagnesemia to the patient’s healthcare provider, if they have been receiving rabeprazole sodium tablets for at least 3 months
- Drug Interactions
Advise patients to report to their healthcare provider if they are taking rilpivirine-containing products [see CONTRAINDICATIONS (4)], warfarin, digoxin or high-dose methotrexate
- Administration
Swallow rabeprazole tablets whole.
- Do not chew, crush or split the tablets. • Do not take this medicine, if you are allergic to Rabeprazole. Signs of an allergic reaction include a rash, itching or shortness of breath.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
- If you have any further questions, ask your doctor or pharmacist.
12. Date of revision of the text
This leaflet was last revised in June 2024.
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What RABIFAST is and what it is used for
- What you need to know before you take RABIFAST
- How to take RABIFAST
- Possible side effects
- How to store RABIFAST
- Contents of the pack and other information
1. What Rabifast is and What It is Used for
The active ingredients of RABIFAST tablets is Rabeprazole, which belong to a group of medicines called Proton Pump Inhibitors (PPIs).
RABIFAST tablets act by reducing the amount of acid made by the stomach.
RABIFAST tablets are used to treat:
- Ulcer in the upper part of the intestine (duodenal ulcer) and benign stomach ulcer.
- Gastro-esophageal reflux disease (GERD) with or without ulcer. GERD is commonly referred to as inflammation of the gullet caused by acid and associated with heartburn. Heartburn is a burning feeling rising from the stomach or lower chest up towards the neck. Rabeprazole tablets may be used as a long term treatment of GERD (GERD maintenance). Rabeprazole tablets may also be used for the symptomatic treatment of moderate to very severe gastro-esophageal reflux disease (symptomatic GERD).
- Zollinger-Ellison Syndrome, which is a condition when the stomach makes extremely high amounts of acid.
2. What You Need to Know Before You Take Rabifast
Do not use RABIFAST if you
- are allergic (hypersensitive) to rabeprazole sodium or any of the other ingredients
- are pregnant or think that you are pregnant
- are breast-feeding.
Do not take RABIFAST tablets if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist before taking RABIFAST tablets.
Warnings and precautions
Talk to your doctor before taking RABIFAST, especially if you
- are allergic to other proton pump inhibitors or “substituted benzimidazoles”.
- have a stomach tumour.
- have or have had any liver problems.
- are taking a medicine called atazanavir (used to treat HIV).
- have reduced body stores or risk factors for reduced vitamin B12 and receive long term treatment with rabeprazole sodium. As with all acid reducing agents, rabeprazole sodium may lead to a reduced absorption of vitamin B12.
- are due to have a specific blood test (Chromogranin A).
- have ever had a skin reaction after treatment with a medicine similar to Rabeprazole that reduces stomach acid.
If you get a rash on your skin, especially in areas exposed to the sun, tell your doctor as soon as you can, as you may need to stop your treatment with RABIFAST. Remember to also mention any other ill-effects like pain in your joints.
If you are not sure if any of the above applies to you, consult your doctor or pharmacist before taking RABIFAST tablets.
Your doctor may perform or have performed an additional investigation called an endoscopy in order to diagnose your condition and/or exclude malignant disease. The possibility of stomach and oesophageal tumours should be excluded before the treatment is started.
If you take RABIFAST tablets on a long-term basis (longer than one year) your doctor will probably monitor you regularly. You should report any new or different symptoms whenever you see your doctor.
Taking a proton pump inhibitor like RABIFAST tablets, especially over a period of more than one year, may slightly increase your risk of fracture of the hip, wrist or spine. Tell your doctor if you have osteoporosis or if you are taking corticosteroids (which can increase the risk of osteoporosis).
Talk to your doctor straight away if you experience severe (watery or bloody) or persistent diarrhoea with symptoms such as fever, abdominal pain or tenderness, as rabeprazole has been associated with a small increase in infectious diarrhoea.
Some abnormal blood and liver enzyme values have been reported during treatment with RABIFAST tablets. Usually, the values become normal when the treatment is discontinued.
Children and adolescents
RABIFAST tablets are not recommended for use in children
Other medicines and RABIFAST
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines obtained without a prescription, including herbal medicines. This is especially important in case you are taking any of the following medicines:
- Atazanavir (used to treat HIV); RABIFAST tablets may lower the amount of this type of medicine in your blood and they should not be used together
- Ketoconazole or itraconazole (used to treat infections caused by a fungus). RABIFAST tablets may lower the amount of this type of medicine in your blood. Your doctor may need to adjust your dose
- Methotrexate (a chemotherapy medicine used in high doses to treat cancer) – if you are taking a high dose of methotrexate, your doctor may temporarily stop your RABIFAST tablets treatment.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before taking RABIFAST tablets.
Pregnancy and breast-feeding
Do not use RABIFAST tablets if you are pregnant or think you may be pregnant.
Do not use RABIFAST tablets if you are breastfeeding or planning to breast-feed.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Occasionally rabeprazole can cause sleepiness. Therefore, driving and operating machinery should be avoided if you are affected.
3.0 How to Use Rabifast
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Taking this medicine
- Only remove a tablet from the blister strip when it is time to take your medicine.
- Swallow your tablets whole with a drink of water.
- Do not chew or crush the tablets.
- Your doctor will tell you how many tablets to take and how long to take them for. This will depend on your condition. When RABIFAST tablets are taken once daily, the tablets should be taken in the morning before breakfast.
- If you are taking this medicine for a long time, your doctor will want to monitor you.
The recommended dose is:
Adults and elderly
Duodenal ulcer: 20mg of Rabeprazole tablets to be taken once daily in the morning. Most patients with duodenal ulcer are treated for four weeks and most patients with benign stomach ulcer are treated for six weeks. However, a few patients may require additional treatment to achieve healing.
Gastro-esophageal Reflux Disease (GERD) with ulcer: 20mg of Rabeprazole tablets to be taken once daily for four to eight weeks.
Long-term treatment of GERD: 20mg of Rabeprazole tablets to be taken once daily up to 4 weeks.
Symptomatic treatment of GERD: 20mg of Rabeprazole tablets once daily for 4 weeks.
Zollinger-Ellison Syndrome: 60mg of Rabeprazole tablets once a day to start with. The dose may then be adjusted by your doctor depending on how you respond to the treatment. Your doctor will tell you how many tablets to take and when to take them.
Use in children
RABIFAST tablets are not recommended for use in children.
If you take more RABIFAST than you should
If you have taken more RABIFAST tablets than you should, talk to a doctor or go to a hospital straight away. Take the medicine pack with you.
If you forget to take RABIFAST
If you forget to take a dose, take it as soon as you remember it. However, if it is almost time for your next dose, skip the missed dose and continue as usual
- If you forget to take your medicine for more than 5 days, talk to your doctor before taking any more medicine
- Do not take a double dose (two doses at the same time) to make up for a forgotten dose.
If you stop taking RABIFAST
Relief of symptoms will normally occur before the ulcer has completely healed. It is important that you do not stop taking the tablets until told to do so by your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects are usually mild and improve without you having to stop taking this medicine.
If you notice any of the following serious side effects, stop taking RABIFAST tablets and contact a doctor immediately, you may need urgent medical treatment:
- Allergic reactions – the signs may include: sudden swelling of your face, difficulty breathing or low blood pressure which may cause fainting or collapse.
- Frequent infections, such as a sore throat or high temperature (fever), or ulcers in your mouth or throat.
- Bruising or bleeding easily.
These side effects are rare (affect fewer than 1 in 1,000 people).
- Sudden onset of severe rash or blistering or peeling skin. This may be associated with a high fever and joint pains (erythema multiforme, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN))
These side effects are very rare (affect fewer than 1 in 10,000 people).
Other possible side effects:
Common side effects (may affect up to 1 in 10 people):
- cough, sore throat (inflammation of the pharynx), runny nose.
- effects on your stomach or gut such as stomach pain, diarrhoea, wind (flatulence), feeling sick (nausea), being sick (vomiting) or constipation
- aches, back pain, non-specific pain.
- weakness or loss of strength, flu like symptoms.
- difficulty sleeping.
- headache, dizziness.
- infection.
- benign polyps in the stomach.
Uncommon side effects (may affect up to 1 in 100 people):
- feeling nervous or drowsy.
- sleepiness.
- chest infection (bronchitis).
- painful and blocked sinuses (sinusitis).
- indigestion, dry mouth, belching.
- rash, skin redness (erythema).
- muscle pains, joint pains, leg cramps.
- bladder infection (urinary tract infection).
- chest pain, chills, fever.
- muscle, leg or joint pain.
- change in how your liver is working (which is measured by blood tests).
- fracture of the hip, wrist or spine.
Rare side effects (may affect up to 1 in 1,000 people):
- blood problems such as reduced number of white cells or platelets. This can cause weakness, bruising or make infections more likely.
- changes in white blood cells (show in blood tests) which may result in frequent infection.
- allergic reactions including facial swelling, low blood pressure and breathing difficulties.
- loss of appetite (anorexia).
- depression.
- visual disturbance.
- upset stomach or stomach pain, sore mouth, taste disturbance.
- inflammation of the liver, jaundice (yellowing of the skin or eyes).
- itchy rash, sweating, skin blisters.
- kidney inflammation (interstitial nephritis).
- increased weight.
Not known (frequency cannot be estimated from the available data):
- low levels of sodium in the blood which can cause tiredness and confusion, muscle twitching, fits and coma.
- confusion.
- swelling of the feet and ankles.
- enlarged breasts in men.
- Patients who have previously had liver problems may very rarely get encephalopathy (a brain disease). • rash, possibly with pain in the joints.
- inflammation of the gut (leading to diarrhoea).
- If you are on Rabeprazole tablets for more than three months it is possible that the levels of magnesium in your blood may fall. Low levels of magnesium can be seen as fatigue, involuntary muscle contractions, disorentation, convulsions, dizziness, increased heart rate. If you get any of these symptoms, please tell your doctor promptly. Low levels of magnesium can also lead to a reduction in potassium or calcium levels in the blood. Your doctor may decide to perform regular blood tests to monitor your levels of magnesium.
Do not be concerned by this list of side effects. You may not get any of them.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to Store Rabifast
- Store in a dry place below 30°C. Protect from light & moisture.
- Keep out of reach of children.
- Tablet should be swallowed whole & not to be broken, crushed or chewed.
- Do not use this medicine after the expiry date which is stated on the blister and the carton after “EXP”. The expiry date refers to the last day of that month.
6. Contents of the Pack and Other Information
What RABIFAST contains
The active substance is Rabeprazole.
Composition:
Rabifast 20
Each gastro-resistant tablet contains
Rabeprazole Sodium IP 20 mg
Excipients q.s.
Colours: Yellow Oxide of Iron & Titanium Dioxide IP
Rabifast 40
Each gastro-resistant tablet contains
Rabeprazole Sodium IP 40 mg
Excipients q.s.
Colours: Ferric Oxide Yellow USPNF & Titanium Dioxide IP
Presentation/pack size
Rabifast 20 Tablets: 10 Blister strips of 15 tablets each
Rabifast 40 Tablets: 10 Blister strips of 10 tablets each
Marketing Authorisation Holder
Zuventus Healthcare Limited
Zuventus House, Plot Y2, CTS No.: 358/A2,
Near Nahur Railway Station,
Nahur (W), Mumbai, 400078 Maharashtra, India.
This leaflet was last revised in 06/2024.