Scavista 12 mg Tablets
Therapy Area
Anti Infective
1.0 Generic Name
Ivermectin Tablets IP 12 mg
2.0 Qualitative and quantitative composition
Each uncoated tablet contains :
Ivermectin IP 12 mg
Excipients q.s.
3.0 Dosage form and strength
Tablet, 12 mg.
4.0 Clinical particulars
4.1 Therapeutic indication
Treatment of intestinal helminths and suppression of microfilaraemia especially with bancrofti infections.
4.2 Posology and method of administration
For the treatment of Strongyloidiasis
Adults : 200 mcg/kg of body weight /dose of ivermectin administered as a single oral dose. In general, additional doses are not necessary. However, follow-up stool examinations should be performed to verify eradication of infection. Patients should take tablets on an empty stomach with water
For the treatment of Bancroft's filariasis caused by Wuchereria bancrofti
Adults : A single dose 200 - 400 mcg/kg body weight ivermectin, given annually for treatment in endemic areas markedly reduces the blood microfilariae count.
For the treatment of ascariasis (roundworm infection)
Adults : 150 to 200 mcg/kg/dose oral as a single dose.
For the treatment of enterobiasis (pinworm infection)
Adults : 200 mcg/kg/dose oral as a single dose; repeat dose in 2 weeks
For the treatment of trichuriasis (whipworm infection)
Adults : 200 mcg/kg/dose oral once daily for 3 days.
4.3 Contraindications
Scavista is contraindicated in patients who are hypersensitive to any component of this product
4.4 Special warnings and precautions for use Warnings
Historical data have shown that microfilaricidal drugs, such as diethylcarbamazine citrate (DEC-C), might cause cutaneous and/or systemic reactions of varying severity (the Mazzotti reaction) and ophthalmological reactions in patients with onchocerciasis. These reactions are probably due to allergic and inflammatory responses to the death of microfilariae. Patients treated with Scavista for onchocerciasis may experience these reactions in addition to clinical adverse reactions possibly, probably, or definitely related to the drug itself. The treatment of severe Mazzotti reactions has not been subjected to controlled clinical trials. Oral hydration, recumbency, intravenous normal saline, and/or parenteral corticosteroids have been used to treat postural hypotension. Antihistamines and/or aspirin have been used for most mild to moderate cases.
Precautions General
After treatment with microfilaricidal drugs, patients with hyperreactive onchodermatitis (sowda) may be more likely than others to experience severe adverse reactions, especially edema and aggravation of onchodermatitis. Rarely, patients with onchocerciasis who are also heavily infected with Loa loa may develop a serious or even fatal encephalopathy either spontaneously or following treatment with an effective microfilaricide. In these patients, the following adverse experiences have also been reported: pain (including neck and back pain), red eye, conjunctival hemorrhage, dyspnea, urinary and/or fecal incontinence, difficulty in standing/walking, mental status changes, confusion, lethargy, stupor, seizures, or coma. This syndrome has been seen very rarely following the use of ivermectin. In individuals who warrant treatment with ivermectin for any reason and have had significant exposure to Loa loa-endemic areas of West or Central Africa, pretreatment assessment for loiasis and careful post-treatment follow-up should be implemented.
4.5 Drugs interactions
Post-marketing reports of increased INR (International Normalized Ratio) have been rarely reported when ivermectin was co-administered with warfarin.
4.6 Use in special populations (such as pregnant women, lactating women, paediatric patients, geriatric patients etc.) Pregnancy, Teratogenic Effects
Pregnancy Category C
Ivermectin has been shown to be teratogenic in mice, rats, and rabbits when given in repeated doses of 0.2, 8.1, and 4.5 times the maximum recommended human dose, respectively (on a mg/m2/day basis). Teratogenicity was characterized in the three species tested by cleft palate; clubbed forepaws were additionally observed in rabbits. These developmental effects were found only at or near doses that were maternotoxic to the pregnant female. Therefore, ivermectin does not appear to be selectively fetotoxic to the developing fetus. There are, however, no adequate and well-controlled studies in pregnant women. Ivermectin should not be used during pregnancy since safety in pregnancy has not been established.
Nursing Mothers
Scavista is excreted in human milk in low concentrations. Treatment of mothers who intend to breast-feed should only be undertaken when the risk of delayed treatment to the mother outweighs the possible risk to the newborn.
Pediatric Use
Safety and effectiveness in pediatric patients weighing less than 15 kg have not been established.
Geriatric Use
Clinical studies of ivermectin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, treatment of an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy
Strongyloidiasis in Immunocompromised Hosts
In immunocompromised (including HIV-infected) patients being treated for intestinal strongyloidiasis, repeated courses of therapy may be required. Adequate and well-controlled clinical studies have not been conducted in such patients to determine the optimal dosing regimen. Several treatments, i.e., at 2-week intervals, may be required, and cure may not be achievable. Control of extra-intestinal strongyloidiasis in these patients is difficult, and suppressive therapy, i.e., once per month, may be helpful.
4.7 Effects on ability to drive and use machines
The effect of Ivermectin on the ability to drive and use machines has not been studied. The possibility in some patients of side effects such as dizziness, somnolence, vertigo and tremor, which may affect the ability to drive or use machines, cannot be excluded.
4.8 Undesirable effects
Strongyloidiasis
In four clinical studies involving a total of 109 patients given either one or two doses of 170 to 200 mcg/kg of ivermectin, the following adverse reactions were reported as possibly, probably, or definitely related to ivermectin:
Body as a Whole : asthenia/fatigue (0.9%), abdominal pain (0.9%)
Gastrointestinal : anorexia (0.9%), constipation (0.9%), diarrhea (1.8%), nausea (1.8%), vomiting (0.9%)
Nervous System/Psychiatric : dizziness (2.8%), somnolence (0.9%), vertigo (0.9%), tremor (0.9%)
Skin : pruritus (2.8%), rash (0.9%), and urticaria (0.9%).
In comparative trials, patients treated with ivermectin experienced more abdominal distention and chest discomfort than patients treated with albendazole. However, ivermectin was better tolerated than thiabendazole in comparative studies involving 37 patients treated with thiabendazole.
The Mazzotti-type and ophthalmologic reactions associated with the treatment of onchocerciasis or the disease itself would not be expected to occur in strongyloidiasis patients treated with ivermectin.
Laboratory Test Findings
In clinical trials involving 109 patients given either one or two doses of 170 to 200 mcg/kg ivermectin, the following laboratory abnormalities were seen regardless of drug relationship: elevation in ALT and/or AST (2%), decrease in leukocyte count (3%). Leukopenia and anemia were seen in one patient.
Onchocerciasis
In clinical trials involving 963 adult patients treated with 100 to 200 mcg/kg ivermectin, worsening of the following Mazzotti reactions during the first 4 days post-treatment were reported: arthralgia/synovitis (9.3%), axillary lymph node enlargement and tenderness (11.0% and 4.4%, respectively), cervical lymph node enlargement and tenderness (5.3% and 1.2%, respectively), inguinal lymph node enlargement and tenderness (12.6% and 13.9%, respectively), other lymph node enlargement and tenderness (3.0% and 1.9%, respectively), pruritus (27.5%), skin involvement including edema, papular and pustular or frank urticarial rash (22.7%), and fever (22.6%).
In clinical trials, ophthalmological conditions were examined in 963 adult patients before treatment, at day 3, and months 3 and 6 after treatment with 100 to 200 mcg/kg ivermectin. Changes observed were primarily deterioration from baseline 3 days post-treatment. Most changes either returned to baseline condition or improved over baseline severity at the month 3 and 6 visits. The percentages of patients with worsening of the following conditions at day 3, month 3 and 6, respectively, were: limbitis: 5.5%, 4.8%, and 3.5% and punctate opacity: 1.8%, 1.8%, and 1.4%. The corresponding percentages for patients treated with placebo were: limbitis: 6.2%, 9.9%, and 9.4% and punctate opacity: 2.0%, 6.4%, and 7.2%. In clinical trials involving 963 adult patients who received 100 to 200 mcg/kg ivermectin, the following clinical adverse reactions were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: facial edema (1.2%), peripheral edema (3.2%), orthostatic
hypotension (1.1%), and tachycardia (3.5%). Drug-related headache and myalgia occurred in <1% of patients (0.2% and 0.4%, respectively). However, these were the most common adverse experiences reported overall during these trials regardless of causality (22.3% and 19.7%, respectively). A similar safety profile was observed in an open study in pediatric patients ages 6 to 13. The following ophthalmological side effects do occur due to the disease itself but have also been reported after treatment with ivermectin: abnormal sensation in the eyes, eyelid edema, anterior uveitis, conjunctivitis, limbitis, keratitis, and chorioretinitis or choroiditis. These have rarely been severe or associated with loss of vision and have generally resolved without corticosteroid treatment.
Laboratory Test Findings
In controlled clinical trials, the following laboratory adverse experiences were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: eosinophilia (3%) and hemoglobin increase (1%).
Post-Marketing Experience for All Indications
The following adverse reactions have been reported since the drug was registered overseas: hypotension (mainly orthostatic hypotension), worsening of bronchial asthma, toxic epidermal necrolysis, Stevens-Johnson syndrome, seizures, elevation of liver enzymes, and elevation of bilirubin.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com Website : http://www.zuventus.co.in/safety.aspx By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Significant lethality was observed in mice and rats after single oral doses of 25 to 50 mg/kg and 40 to 50 mg/kg, respectively. No significant lethality was observed in dogs after single oral doses of up to 10 mg/kg. At these doses, the treatment-related signs that were observed in these animals include ataxia, bradypnea, tremors, ptosis, decreased activity, emesis, and mydriasis. In accidental intoxication with, or significant exposure to, unknown quantities of veterinary formulations of ivermectin in humans, either by ingestion, inhalation, injection, or exposure to body surfaces, the following adverse effects have been reported most frequently: rash, edema, headache, dizziness, asthenia, nausea, vomiting, and diarrhea. Other adverse effects that have been reported include: seizure, ataxia, dyspnea, abdominal pain, paresthesia, urticaria, and contact dermatitis. In case of accidental poisoning, supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present. Induction of emesis and/or gastric lavage as soon as possible, followed by purgatives and other routine anti-poison measures, may be indicated if needed to prevent absorption of ingested material.
5.0 Pharmacological properties
5.1 Mechanism of Action
Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
5.2 Pharmacodynamic properties
Ivermectin is a member of the avermectin class of broad-spectrum antiparasitic agents which have a unique mode of action. The selective activity of compounds of this class is attributable to the facts that some mammals do not have glutamate-gated chloride channels and that the avermectins have a low affinity for mammalian ligand-gated chloride channels. In addition, ivermectin does not readily cross the blood-brain barrier in humans. Ivermectin is active against various life-cycle stages of many but not all nematodes. It is active against the tissue microfilariae of Onchocerca volvulus but not against the adult form. Its activity against Strongyloides stercoralis is limited to the intestinal stages.
5.3 Pharmacokinetic properties
Following oral administration of ivermectin, plasma concentrations are approximately proportional to the dose. In two studies, after single 12-mg doses of ivermectin in fasting healthy volunteers (representing a mean dose of 165 mcg/kg), the mean peak plasma concentrations of the major component (H2B1a) were 46.6 (±21.9) (range: 16.4-101.1) and 30.6 (±15.6) (range: 13.9-68.4) ng/mL, respectively, at approximately 4 hours after dosing. Ivermectin is metabolized in the liver, and ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The plasma half-life of ivermectin in man is approximately 18 hours following oral administration. The safety and pharmacokinetic properties of ivermectin were further assessed in a multipledose clinical pharmacokinetic study involving healthy volunteer. Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) ivermectin following a standard high-fat (48.6 g of fat) meal. Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5 fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of ivermectin.
vermectin was not genotoxic in vitro in the Ames microbial mutagenicity assay of Salmonella typhimurium strains TA1535, TA1537, TA98, and TA100 with and without rat liver enzyme activation, the Mouse Lymphoma Cell Line L5178Y (cytotoxicity and mutagenicity) assays, or the unscheduled DNA synthesis assay in human fibroblasts.
Ivermectin had no adverse effects on the fertility in rats in studies at repeated doses of up to 3 times the maximum recommended human dose of 200 mcg/kg (on a mg/m2/day basis).
7.0 Description
Scavista (Ivermectin) is a semisynthetic, anthelmintic agent for oral administration. Ivermectin is derived from the avermectins, a class of highly active broad-spectrum, antiparasitic agents isolated from the fermentation products of Streptomyces avermitilis. Ivermectin is a mixture containing at least 90% 5-Odemethyl-22,23-dihydroavermectin A1a and less than 10% 5-O-demethyl-25-de(1-methylpropyl)-22,23-dihydro25-(1- methylethyl)avermectin A1a, generally referred to as 22,23-dihydroavermectin B1a and B1b, or H2B1a and H2B1b, respectively. The respective empirical formulas are C48H74O14 and C47H72O14, with molecular weights of 875.10 and 861.07, respectively. The structural formulas are :
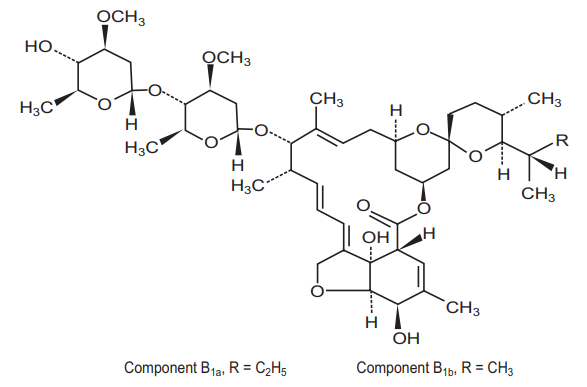
Ivermectin is a white to yellowish-white, nonhygroscopic, crystalline powder with a melting point of about 155°C. It is insoluble in water but is freely soluble in methanol and soluble in 95% ethanol.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not Applicable
8.2 Shelf-life
24 months
8.3 Packaging information
A blister strip of 2 tablets.
8.4 Storage and handing instructions
Store protected from moisture, at a temperature not exceeding 30°C.
Keep out of reach of children.
9.0 Patient Counselling Information
Scavista tablet should be taken on an empty stomach with water. Strongyloidiasis : The patient should be reminded of the need for repeated stool examinations to document clearance of infection with Strongyloides stercoralis. Onchocerciasis : The patient should be reminded that treatment with Scavista does not kill the adult Onchocerca parasites, and therefore repeated follow-up and retreatment is usually required.