Setolac®-p
Therapy Area
Pain management
1.0 Name of the medicinal product
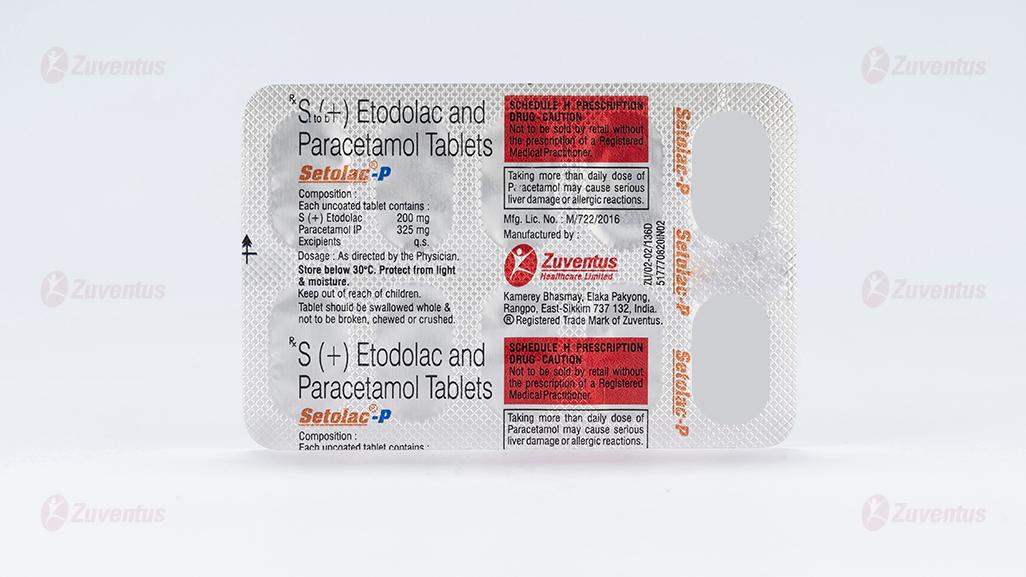
S (+) Etodolac and Paracetamol Tablets (200mg + 325mg)
2.0 Qualitative and quantitative composition
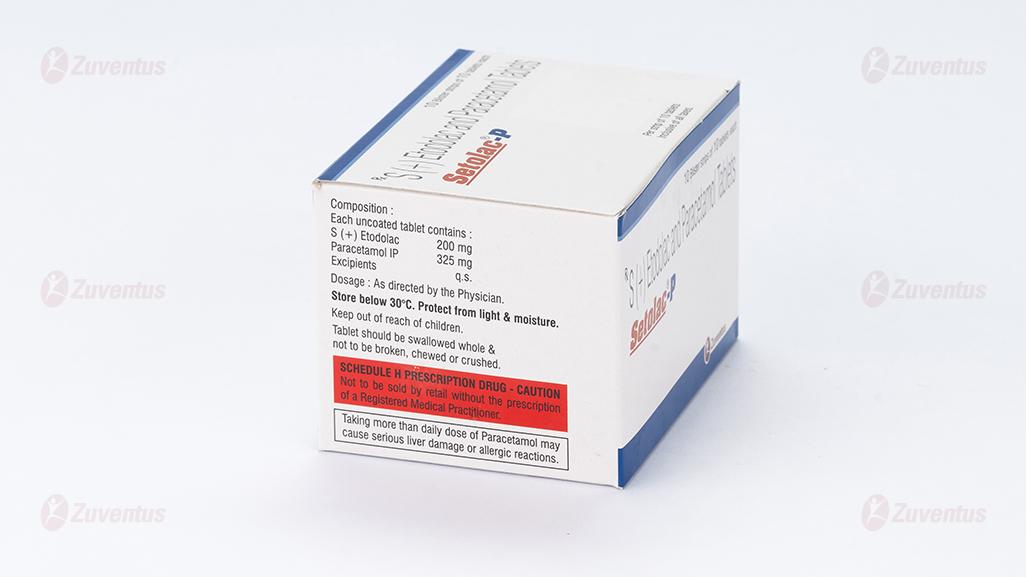
Each uncoated tablet contains:
S (+) Etodolac 200 mg
Paracetamol IP 325 mg
Excipients q.s
3.0 Dosage form and strength
Tablet
200 mg of S (+) Etodolac and 325 mg of Paracetamol
4.0 Clinical Particulars
4.1 Therapeutic indication
For the symptomatic treatment of acute pain and inflammation in patients with osteoarthritis, rheumatoid arthritis and ankylosing spondylitis.
4.2 Posology and method of administration
Adults: One tablet two times daily orally.
Paediatric population: Not recommended.
Method of administration: For oral administration.
To be taken preferably with or after food. Swallow the tablet whole with a glass of water.
4.3 Contraindications
- Patients with known hypersensitivity to S (+) Etodolac and/or Paracetamol.
- Patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs.
- Patients with severe hepatocellular insufficiency
- Setolac®-P Tablets should not be used in patients with active or history of recurrent peptic ulceration or a history of peptic ulcer disease (with two or more distinct episodes of proven ulceration or bleeding).
- During the last trimester of pregnancy.
- Patients with hepatic failure, active liver disease and renal failure.
- Setolac®-P tablet is not recommended for pediatric use.
4.4 Special warnings and precautions for use
Undesirable effects may be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms.
The use of Setolac®-P Tablets with concomitant NSAIDs including cyclooxygenase-2-selective inhibitors should be avoided.
Cardiovascular and Cerebrovascular Effects
COX-2 selective and non-selective NSAIDs have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV events in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. NSAIDs, including Etodolac, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy. Fluid retention and edema have been observed in some patients taking NSAIDs. Setolac®-P tablets should be used with caution in patients with fluid retention or heart failure.
Pre-existing Asthma
Patients with asthma may have Aspirin-sensitive asthma. Since cross reactivity, including bronchospasm, between Aspirin and other NSAIDs has been reported in such Aspirin-sensitive patients, Setolac®-P tablets should not be administered to patients with this form of Aspirin sensitivity and should be used with caution in patients with pre-existing asthma.
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. In these patients, administration of a NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. No information is available from controlled clinical studies regarding the use of S (+) Etodolac extended-release tablets in patients with advanced renal disease. Therefore, treatment with Setolac®-P tablets is not recommended in these patients with advanced renal disease.
Hepatic Effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including S (+) Etodolac. Owing to the presence of Paracetamol, Setolac®-P tablets should be used with caution in patients with hepatocellular insufficiency or non-cirrhotic alcoholic liver disease. Impairment of renal or hepatic functions due to other causes may alter drug metabolism; patients receiving concomitant long term therapy, especially the elderly, should be observed for potential side effects and their drug doses adjusted as needed, or the drug discontinued.
Gastrointestinal Effects
NSAIDs, including Etodolac, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. Setolac®-P tablets should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. To minimize the potential risk for an adverse GI event in patients, the lowest effective dose should be used for the shortest possible duration. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including Etodolac, due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including Etodolac, should have their hemoglobin or haematocrit checked if they exhibit any signs or symptoms of anemia. NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Patients receiving Setolac®-P tablets who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
SLE and mixed connective tissue disease:
In patients with systemic lupus erythematous (SLE) and mixed connective tissue disorders there may be an increased risk of aseptic meningitis.
Dermatological:
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs (see section 4.8). Patients appear to be at highest risk for these reactions early in the course of therapy: the onset of the reaction occurring in the majority of cases within the first month of treatment. Tablets should be discontinued at the first appearance of the skin rash, mucosal lesions, or any other sign of hypersensitivity
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to Etodolac. Setolac®-P tablets should not be given to patients with the Aspirin triad.
Other Precautions
- S (+) Etodolac cannot be expected to substitute corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation.
- The pharmacological activity of Setolac®-P in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
- Setolac®-P tablets should be used with caution in patients with severe renal insufficiency (creatinine clearance 30 mL/min), Glucose-6-Phosphate Dehyrogenase (G6PD) deficiency, chronic alcoholism, excessive alcohol intake (3 or more alcoholic drinks every day), anorexia, bulimia or cachexia; chronic malnutrition (low reserves of hepatic glutathione), dehydration, and hypovolemia.
4.5 Drug interactions
ACE inhibitors: Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors.
Aspirin: When Etodolac is administered with Aspirin, its protein binding is reduced, although the clearance of free Etodolac is not altered.
Busulfan: Busulfan is eliminated from the body via conjugation with Glutathione. Concomitant use with Paracetamol may result in reduced Busulfan clearance.
Other analgesics including cyclooxygenase-2 selective inhibitor: Avoid concomitant use of two or more NSAIDs (including aspirin) as this may increase the risk of adverse effects (see section 4.4)
Anti-hypertensives: Reduced anti-hypertensive effect
Diuretics: Etodolac can reduce the natriuretic effect of furosemide and thiazides in some patients with possible loss of blood pressure control. Caution should be paid to the concomitant intake of enzyme-inducing agents. Induction of metabolism of Paracetamol from enzyme inducers may result in an increased level of hepatotoxic metabolites.
Cardiac glycosides: NSAIDs may exacerbate cardiac failure, reduce GFR and increase plasma glycoside levels.
Lithium: NSAIDs have produced an elevation of plasma Lithium levels and a reduction in renal Lithium clearance. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and Lithium are administered concurrently, subjects should be observed, carefully for signs of Lithium toxicity.
Cyclosporin, Digoxin, Methotrexate: Etodolac, like other NSAIDs, through effects on renal prostaglandins, may cause changes in the elimination of these drugs leading to elevated serum levels of Cyclosporine, Digoxin, Methotrexate, and increased toxicity. Nephrotoxicity associated with Cyclosporine may also be enhanced. Concomitant diflunisal increases Paracetamol plasma concentrations and this may increase hepatotoxicity.
Anti-coagulants: NSAIDs may enhance the effects of anti-coagulants, such as warfarin (see section 4.4)
Anti-platelet agents and selective serotonin reuptake inhibitors (SSRIs): Increased risk of gastrointestinal bleeding (see section 4.4)
Tacrolimus: Possible increased risk of nephrotoxicity when NSAIDs are given with tacrolimus.
Zidovudine: Increased risk of haematological toxicity when NSAIDs are given with zidovudine. There is an evidence of an increased risk of haemarthroses and haemtoma in HIV (+) haemophiliacs receiving concurrent treatment with zidovudine and ibuprofen.
Bilirubin tests can give a false positive result due to the presence of phenolic metabolites of Setolac®-P Tablets in the urine.
Mifepristone: NSAIDs should not be used for 8-12 days after mifepristone administration as NSAIDs can reduce the effect of mifepristone.
Corticosteroids: increased risk of gastrointestinal ulceration or bleeding (see section 4.4)
Quinolone antibiotics: animal data indicate that NSAIDs can increase the risk of convulsions associated with quinolone antibiotics. Patients taking NSAIDs and quinolones may have an increased risk of developing convulsions.
Phenylbutazone: Phenylbutazone causes increase (by about 80%) in the free fraction of Etodolac though the clinical implication of the same is not known.
Cholestyramine: The speed of absorption of paracetamol is reduced by cholestyramine. Therefore, the cholestyramine should not be taken within one hour if maximal analgesia is required.
Metoclopramide and Domperidone: The absorption of paracetamol is increased by metoclopramide and domperidone. However, concurrent use need not be avoided.
Warfarin: The anticoagulant effect of warfarin and other coumarins may be enhanced by prolonged regular use of paracetamol with increased risk of bleeding; occasional doses have no significant effect.
Chloramphenicol: Increased plasma concentration of chloramphenicol.
Phenytoin: Administered concomitantly may result in decreased Paracetamol effectiveness and an increased risk of hepatotoxicity.
Probenecid: Causes an almost 2-fold reduction in clearance of Paracetamol by inhibiting its conjugation with Glucuronic acid.
Salicylamide: May prolong the amount of time Paracetamol stays in the body.
4.6 Use in special populations
Fertility:
The use of Setolac®-P Tablets may impair female fertility and is not recommended in woman attempting to conceive. In women who have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of Setolac®-P Tablets should be considered.
Pregnancy:
In teratology studies, isolated occurrences of alterations in limb development were found and included polydactyly, oligodactyly, syndactyly, and unossified phalanges in rats and oligodactyly and synostosis of metatarsals in rabbits. Drugs which inhibit prostaglandin biosynthesis may cause dystocia and delayed parturition as evidenced by studies in pregnant animals. Congenital abnormalities have been reported in association with NSAID administration in man; however, these are low in frequency and do not appear to follow any discernible pattern. In view of the known effects of NSAIDs on the foetal cardiovascular system, some inhibitors of prostaglandin biosynthesis have been shown to interfere with the risk of closure of the ductus arteriosus, use in the last trimester of pregnancy is contraindicated. The onset of labour may be delayed and the duration increased with an increased bleeding tendency in both mother and child.
From the 20th week of pregnancy onward, etodolac use may cause oligohydramnios resulting from foetal renal dysfunction. This may occur shortly after treatment initiation and is usually reversible upon discontinuation. In addition, there have been reports of ductus arteriosus constriction following treatment in the second trimester, most of which resolved after treatment cessation. Therefore, during the first and second trimester of pregnancy, etodolac should not be given unless clearly necessary. If etodolac is used by a woman attempting to conceive, or during the first and second trimester of pregnancy, the dose should be kept as low and duration of treatment as short as possible. Antenatal monitoring for oligohydramnios and ductus arteriosus constriction should be considered after exposure to etodolac for several days from gestational week 20 onward. Etodolac should be discontinued if oligohydramnios or ductus arteriosus constriction are found.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the foetus to:
- cardiopulmonary toxicity (premature constriction/closure of the ductus arteriosus and pulmonary hypertension);
- renal dysfunction (see above); the mother and the neonate, at the end of pregnancy, to:
- possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses.
- inhibition of uterine contractions resulting in delayed or prolonged labour.
Consequently, etodolac is contraindicated during the third trimester of pregnancy.
Lactation:
It is not known whether Setolac®-P is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from S (+) Etodolac, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother
Pediatric use:
Safety and effectiveness in pediatric patients below the age of 18 years have not been established.
Geriatric use:
As with any NSAID, caution should be exercised in treating the elderly (65 years and older) and when increasing the dose. Elderly patients may be more sensitive to the anti-prostaglandin effects of NSAIDs (on the gastrointestinal tract and kidneys) than younger patients. In particular, elderly or debilitated patients who receive NSAID therapy seem to have a lower tolerance for gastrointestinal ulceration or bleeding as compared to other individuals, and most spontaneous reports of fatal GI events are in this population. S (+) Etodolac is eliminated primarily by the kidney. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
4.7 Effects on ability to drive and use machines
Setolac®-P may cause dizziness. So, you should not drive or operate heavy machinery if you feel dizzy or not fully alert.
4.8 Undesirable effects
Most frequently reported adverse reactions (approx. 1 - 10%):
Gastrointestinal experiences including: abdominal pain, constipation, diarrhea, dyspepsia, flatulence, gross bleeding / perforation, heartburn, nausea, GI ulcers (gastric / duodenal), vomiting.
Other events including: abnormal renal function, anemia, dizziness, edema, elevated liver enzymes, headaches, increased bleeding time, pruritis, rashes, tinnitus.
Additional NSAID Adverse Experiences Reported Occasionally with Body as a whole: Allergic reaction, anaphylactic / anaphylactoid reactions (including shock), chills, fever, sepsis.
Digestive system: Anorexia, cholestatic hepatitis / jaundice, dry mouth, duodenitis, esophagitis, gastritis, gastric peptic ulcers, glossitis, hepatic failure, hepatitis, hematemesis, intestinal ulceration, jaundice, liver necrosis, melena, pancreatitis, rectal bleeding, stomatitis. Less frequently, gastritis has been observed. Pancreatitis has been reported very rarely.
Hypersensitivity: Hypersensitivity reactions have been reported following treatment with NSAIDs. These may consist of (a)non-specific allergic reactions and anaphylaxis (b)respiratory tract reactivity comprising asthma, aggravated asthma, bronchospasm or dyspnoea, or (c)assorted skin disorders, including rashes of various types, pruritus, urticaria, purpura, angioedema and more rarely exfoliative and bullous dermatoses (including epidermal necrolysis and erythema multiforme).
Cardiovascular and cerebrovascular:
Congestive heart failure, flushing, palpitations, tachycardia, syncope, vasculitis (including necrotizing and allergic). Oedema, hypertension and cardiac failure have been reported in association with NSAID treatment Clinical trial and epidemiological data suggest that use of some NSAIDs (particularly at high doses and in long term treatment) may be associated with an increased risk of arterial thrombotic events (for example myocardial infarction or stroke).
Hepatic:
Abnormal liver function, hepatitis and jaundice Metabolic and nutritional:
Hyperglycemia in previously controlled diabetic patients.
Neurological and special senses: Visual disturbances, photophobia, blurred vision, optic neuritis, headaches, paraethesia, reports of aseptic meningitis (especially in patients with existing auto-immune disorders, such as systemic lupus erythematous, mixed connective tissue disease), with symptoms such as stiff neck, headache, nausea, vomiting, fever or disorientation (See section 4.4), depression, confusion, hallucinations, tinnitus, vertigo, dizziness, malaise, fatigue and drowsiness. Anxiety, confusion, depression, dream abnormalities, insomnia, nervousness, paresthesia, somnolence, tremors, vertigo.
Blood and lymphatic system:
Agranulocytosis, ecchymosis, eosinophilia, hemolytic anemia, leukopenia, neutropenia, pancytopenia, purpura, thrombocytopenia and aplastic anaemia. There have been reports of blood dyscrasias including methaemoglobenaemia and agranulocytosis, but these were not necessarily causality related to paracetamol.
Respiratory system:
Asthma, dyspnea, pulmonary infiltration with eosinophilia.
Dermatological:
Bullous reactions including Stevens Johnson Syndrome and Toxic Epidermal Necrolysis (very rare). Photosensitivity. Angioedema, cutaneous vasculitis with purpura, erythema multiforme, hyperpigmentation, sweating, urticaria, vesiculobullous rash. Fixed drug eruption (FDE) is associated with Paracetamol use.
Urogenital system:
Dysuria, elevated BUN, oliguria / polyuria, proteinuria, renal failure, renal insufficiency, renal papillary necrosis, serum creatinine increase, urinary frequency, Nephrotoxicity in various forms, including interstitial nephritis, nephrotic syndrome.
Reporting of side effects
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Etodolac
Symptoms include headache, nausea, vomiting, epigastric pain, gastro-intestinal bleeding, rarely diarrhoea, disorientation, excitation, coma, drowsiness, dizziness, tinnitus, fainting, occasionally convulsions. In cases of significant poisoning acute renal failure and liver damage are possible.
Management: Patients should be treated symptomatically as required. Within one hour of ingestion of a potentially toxic amount, activated charcoal should be considered. Alternatively, in adults, gastric lavage should be considered within one hour of indigestion of a potentially life-threatening overdose. Good urine output should be ensured. Renal and liver function should be closely monitored. Patients should be observed for at least four hours after ingestion of potentially toxic amounts. Frequent or prolonged convulsions should be treated with intravenous diazepam. Other measures may be indicated by the patient's clinical condition. The standard practices of gastric lavage, activated charcoal administration and general supportive therapy should be undertaken.
Paracetamol
Symptoms of paracetamol over dosage in the first 24 hours are pallor, nausea, vomiting, anorexia and abdominal pain. Liver damage may become apparent 12 to 48 hours after ingestion. Abnormalities of glucose metabolism and metabolic acidosis may occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose. In severe poisoning, hepatic failure may progress to encephalopathy, haemorrhage, hypoglycaemia, cerebral oedema, and death. Acute renal failure with acute tubular necrosis, strongly suggested by loin pain, haematuria and proteinuria, may develop even in the absence of severe liver damage. Cardiac arrhythmias and pancreatitis have been reported.
Management: Immediate treatment is essential in the management of paracetamol overdose. Despite a lack of significant early symptoms, patients should be referred to hospital urgently for immediate medical attention. Symptoms may be limited to nausea or vomiting and may not reflect the severity of overdose or the risk of organ damage.
Treatment with activated charcoal should be considered if the overdose has been taken within 1 hour. Plasma paracetamol concentration should be measured at 4 hours or later after ingestion (earlier concentrations are unreliable). Treatment with N-acetylcysteine may be used up to 24 hours after ingestion of paracetamol however, the maximum protective effect is obtained up to 8 hours post ingestion. If required the patient should be given intravenous-N-acetylcysteine, in line with the established dosage schedule. If vomiting is not a problem, oral methionine may be a suitable alternative for remote areas, outside hospital.
Management of patients who present with serious hepatic dysfunction beyond 24 hours from ingestion should be discussed with the NPIS or a liver unit
5.0 Pharmacological Properties
5.1 Mechanism of Action
S (+) Etodolac is the pharmacologically active component of racemate Etodolac. The anti-inflammatory, analgesic, and antipyretic activities of S (+) Etodolac have been observed to be due to inhibition of cyclooxygenase-2 (COX-2) resulting in inhibition of prostaglandin synthesis.
The precise mechanism of the analgesic and antipyretic properties of Paracetamol has yet to be established. It acts both centrally as well as peripherally. Paracetamol is an antipyretic with good analgesic property.
5.2 Pharmacodynamic properties
Etodolac
Inhibition of prostaglandin synthesis and COX-2 selectivity: All non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to inhibit the formation of prostaglandins. It is this action which is responsible both for their therapeutic effects and some of their side-effects. The inhibition of prostaglandin synthesis observed with etodolac differs from that of other NSAIDs. In an animal model at an established anti-inflammatory dose, cytoprotective PGE concentration in the gastric mucosa have been shown to be reduced to a lesser degree and for a shorter period than other NSAIDs. This finding is consistent with subsequent in-vitro studies which have found etodolac to be selective for induced cyclo-oxygenase 2 (COX-2, associated with inflammation) over COX-1 (cytoprotective).
Furthermore, studies in human cell models have confirmed that etodolac is selective for the inhibition of COX-2.
The clinical benefit of preferential COX-2 inhibition over COX-1 has yet to be proven.
Anti-inflammatory effects: Experiments have shown etodolac to have marked anti-inflammatory activity, being more potent than several clinically established NSAIDs.
Paracetamol
Analgesic – The mechanism of analgesic action has not been fully determined. Paracetamol may act predominantly by inhibiting prostaglandin synthesis in the central nervous system (CNS) and to a lesser extent, through a peripheral action by blocking pain-impulse generation. The peripheral action may also be due to inhibition of prostaglandin synthesis or to inhibition of the synthesis or actions of other substances that sensitise pain receptors to mechanical or chemical stimulation.
Antipyretic – Paracetamol probably produces anti-pyresis by acting centrally on the hypothalamic heat-regulation centre to produce peripheral vasodilation resulting in increased blood flow through the skin, sweating and heat loss. The central action probably involves inhibition of prostaglandin synthesis in the hypothalamus.
5.3 Pharmacokinetic properties
S (+) Etodolac is well absorbed and does not undergo significant first-pass metabolism following oral administration. The extent of absorption of S (+) Etodolac is not affected after a meal. Food intake, however, reduces the peak concentration reached by approximately one-half and increases the time to peak concentration.
Paracetamol is well absorbed from the gastrointestinal tract in humans. The ingestion of food along with Paracetamol does not seem to affect its absorption.
| Parameters | S (+) Etodolac | Paracetamol |
| Oral bioavailability | 80% | 63 – 89% |
| Cmax | 4.07 µg/ml | 12.3 ng/mL |
| Tmax | 3.3 hr | 1.1 hr |
| Vd | Approx. 390 mL/kg | 51 L |
| Plasma protein binding | More than 99% | 10% to 25% |
| Half-life | 6.2 hr | 2 hr |
| Metabolism | S (+) Etodolac is extensively metabolized in the liver. The metabolites include 6-, 7-, and 8-hydroxylated-etodolac and Etodolac glucuronide. | Metabolism is primarily in the liver, via conjugation with Glucuronic (60%) and Sulphuric (35%) acids, or Cysteine (3%). A small amount of drug undergoes cytochrome P-450 mediated N-hydroxylation to form N-acetylp-aminobenzoquino-neimine. |
| Excretion | Approx. 72% of the dose excreted into urine as parent drug plus metabolite. Fecal excretion accounts for 16% of the dose. | 90 - 100% of the drug is recoverable in the urine as conjugates |
6.0 Nonclinical Properties
6.1 Animal Toxicology or Pharmacology
Etodolac: Not Available
Paracetamol: Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development. Conventional studies using the currently accepted standards for the evaluation of toxicity to reproduction and development are not available
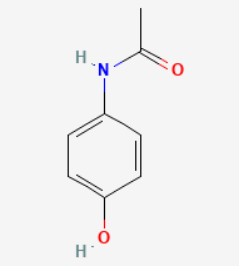
7.0 Description
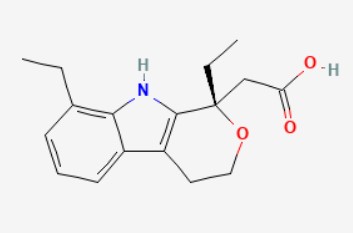
(S)-etodolac is the S-enantiomer of etodolac. It is a preferential inhibitor of cyclo-oxygenase 2 and a non-steroidal anti-inflammatory, whereas the enantiomer, (R)-etodolac, is inactive. The racemate is commonly used for the treatment of rheumatoid arthritis and osteoarthritis, and for the alleviation of postoperative pain. Chemical Name: 2-[(1S)-1,8-diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl]acetic acid Molecular Formula: C17H21NO3 Molecular Weight: 287.35 g/mol
Structure:
Paracetamol is a well-established analgesic.
Chemical Name: N-(4-hydroxyphenyl)acetamide
Molecular Formula: C8H9NO2
Molecular Weight: 151.2 g/mol
Structure:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf life
Refer on the pack.
8.3 Packaging Information
PVC-Alu blister strip of 10 tablets
8.4 Storage and handling instructions
Store below 30ºC. Protect from light and moisture. Keep out of reach of children. Tablet should be swallowed whole & not to be broken, chewed or crushed.
Any unused product or waste material should be disposed of in accordance with local requirements.
9.0 Patient Counselling Information
- Take it with food to avoid getting an upset stomach.
- The drug should be taken precisely as it has been prescribed. Meant for oral consumption, Setolac®-P Tablet should be taken whole. Do not chew or crush it as it may reduce its effect on the body.
- It is best that you do not stop the medication mid-course as the pain may reoccur. Complete the entire treatment course for the best results.
- Avoid consuming alcohol when taking Setola®-P Tablet as it may cause excessive drowsiness and increase the risk of liver damage.
- Do not take it with any other medicine containing acetaminophen (drugs for pain / fever or cough-and-cold) without asking your doctor first.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again. If you have any further questions, please ask your doctor or pharmacist. This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. If you get any side effects, talk to you doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1.What Setolac®-P Tablets is and what it is used for
2.What you need to know before you take Setolac®-P Tablets
3.How to take Setolac®-P Tablets
4.Possible side effects
5.How to store Setolac®-P Tablets
6.Contents of the pack and other information
1. What Setolac®-P Tablets is and what it is used for
Setolac®-P is one of a group of medicines called "non-steroidal anti-inflammatory drugs" (NSAIDs) which are usually taken to relieve the pain, stiffness, inflammation and swelling which is often associated with arthritis.
Setolac®-P Tablets is used to treat the symptoms of rheumatoid arthritis, osteoarthritis and ankylosing spondylitis by reducing inflammation, swelling, stiffness, and joint pain.
Each tablet contains 200mg of the S (+) Etodolac and 325mg of Paracetamol. S (+) Etodolac is the pharmacologically active component of racemate Etodolac. S (+) Etodolac has anti-inflammatory, analgesic, and antipyretic activities. Paracetamol works by relieving pain and reducing high temperature and fever. It is for effective relief from: mild to moderate pain including headache, migraine, sharp nerve pain (neuralgia), toothache, sore throat, period pains, aches and pain, symptomatic relief of rheumatic aches and pains, influenza symptoms, feverishness and feverish colds.
2. What you need to know before you take Setolac®-P Tablets
DO NOT take Setolac®-P Tablets if you:
- If you are allergic to etodolac or paracetamol or any of the other ingredients of this medicine (listed in section 6)
- If you have severe heart failure
- If you have a peptic ulcer (a small erosion or hole in the stomach or duodenum) or bleeding in your stomach, or have had two or more episodes of peptic ulcers, stomach bleeding or perforation.
- If you have had an allergic reaction or asthmatic type reaction (e.g., wheezing, itching or skin rash) after taking aspirin, Setolac®-P Tablets or another NSAID
- If you have hepatic failure and renal failure
- If you are pregnant, think you may be pregnant or are breast feeding
- If you have severe hepatocellular insufficiency
Warnings and Precautions
Talk to your doctor or pharmacist before taking Setolac®-P Tablets.
- If you suffer from kidney, heart or liver disease (including alcoholic liver disease), or a blood disorder, especially if you are also taking diuretics (water tablets). The dose should be as low as possible and you should have regular checks
- If you are already on long-term therapy with a medicine other than Setolac®-P Tablets, since your doctor will want to arrange regular check-ups, especially if you are elderly
- If you suffer from fluid retention (swelling of legs, ankles or feet)
- If you suffer from high blood pressure or heart failure
- If you suffer from, or have ever suffered from, asthma or breathing difficulties
- If you have heart problems, previous stroke or think that you might be at risk of these conditions (for example if you have high blood pressure, diabetes or high cholesterol or are a smoker)
- If you suffer from G-6-PD deficiency (a hereditary condition leading to low red blood cell counts).
Medicines such as Setolac®-P Tablets may be associated with a small increased risk of heart attack (myocardial infarction) or stroke. Any risk is more likely with high doses and prolonged treatment. Do not exceed the recommended dose or duration of treatment.
If you have heart problems, previous stroke or think that you might be at risk of these conditions (for example if you have high blood pressure, diabetes or high cholesterol or are a smoker) you should discuss your treatment with your doctor or pharmacist.
Serious gastrointestinal side effects such as bleeding, ulceration and perforation can occur at any time with or without warning symptoms in patients treated with NSAIDs. If any sign of gastrointestinal bleeding occurs, Setolac®-P Tablets should be stopped immediately.
Children
Setolac®-P Tablets is not recommended for use in children.
Other medicines and Setolac®-P Tablets
Tell your doctor if you are taking, have recently taken or might take any other medicines. Tell your doctor if you are taking:
- Warfarin - for blood thinning
- Cyclosporin - following transplantation
- Digoxin - for heart problems
- Lithium - for mental illness
- Methotrexate - used to treat conditions such as psoriasis or rheumatoid arthritis
- Corticosteroids (such as prednisolone)
- Quinolone antibiotics e.g. ciprofloxacin
- Aspirin
- Other NSAIDs - ibuprofen, naproxen, diclofenac
- Drugs to control high blood pressure
- Mifepristone (a drug to induce abortion) in the last 12 days
- Warfarin
- Antibiotics (chloramphenicol)
- anti-sickness treatments (metoclopramide, domperidone)
- treatments for diarrhoea caused by gall bladder disease
- lipid lowering drugs (cholestyramine).
If you have a blood or urine test, tell your doctor that you are taking Setolac®-P Tablets, as the drug may affect the results.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. You should inform your doctor if you have problems becoming pregnant. NSAIDs may make it more difficult to become pregnant.
Do not take Setolac®-P Tablets if you are in the last 3 months of pregnancy as it could harm your unborn child or cause problems at delivery. It can cause kidney and heart problems in your unborn baby. It may affect your and your baby’s tendency to bleed and cause labour to be later or longer than expected. You should not take Setolac®-P Tablets during the first 6 months of pregnancy unless absolutely necessary and advised by your doctor. If you need treatment during this period or while you are trying to get pregnant, the lowest dose for the shortest time possible should be used. If taken for more than a few days from 20 weeks of pregnancy onward, Setolac®-P Tablets can cause kidney problems in your unborn baby that may lead to low levels of amniotic fluid that surrounds the baby (oligohydramnios) or narrowing of a blood vessel (ductus arteriosus) in the heart of the baby. If you need treatment for longer than a few days, your doctor may recommend additional monitoring.
Setolac®-P Tablets should not be used if you are breast-feeding. It is not known if this medicine passes into breast milk. It is not recommended for use during breast-feeding unless considered essential by your doctor.
Driving and using machines
Setolac®-P may cause dizziness. So, you should not drive or operate heavy machinery if you feel dizzy or not fully alert.
3. How to take Setolac®-P Tablets
Always take Setolac®-P Tablets exactly as your doctor told you. Check with your doctor or pharmacist if you are not sure. Check the pharmacist's label for the dose recommended for you.
The recommended adult dose is one Setolac®-P tablet twice a day.
The tablet should be swallowed whole with a glass of water. Take with or after food. Do not chew or crush the tablet. Setolac®-P Tablets is not recommended for use in children.
If you take more Setolac®-P Tablets than you should
If you take more tablets than you should (an overdose), seek medical attention immediately. Always take the bottle (or packaging) with you, even if empty. Symptoms of overdose include headache, feeling sick, vomiting, stomach pain, passing blood in faeces or passing black tarry stools. On rare occasions diarrhoea, disorientation, excitation, coma, drowsiness, dizziness, ringing in the ears, fainting, and convulsive fitting may occur. In cases of significant overdose kidney failure and liver damage are possible. In some cases, irregular heartbeat and inflammation of the pancreas is also reported.
If you forget to take Setolac®-P Tablets
Do not take a double dose to make up for the forgotten dose. Take your tablet as soon as you remember and continue to take your medicine as usual, but do not take more than one tablet a day.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most serious side effects that may occur with Setolac®-P Tablets are serious allergic or hypersensitivity reactions, heart failure, stroke, kidney failure, liver failure, inflammation of the pancreas and aseptic meningitis. If you suffer from any of the symptoms described below: stop taking Setolac®-P Tablets and call a doctor straight away.
Allergic or hypersensitivity reactions may have the following symptoms: wheezing, difficulty breathing or shortness of breath, swelling of the face, lips, mouth or tongue extensive rash, peeling or blistering of the skin, continuous itching.
Heart and blood circulatory disorders symptoms: Chest pain, high blood pressure, swelling of the ankles, palpitations (throbbing of heart), several types of anaemia or other blood disorders, unexpected bruising and bleeding.
Stomach and bowel (gastrointestinal) problems: If you Pass blood in your faeces (stools/motions) Pass black tarry stools. Vomit any blood or dark particles that look like coffee grounds.
Kidney failure symptoms: Difficulty or pain when passing urine, discolouration of urine or urinating more or less often than usual.
Liver failure and inflammation of the pancreas symptoms: Jaundice (yellowing of the eyes or skin), abdominal pain, abnormal liver function test results.
Aseptic Meningitis symptoms
A serious rare condition known as aseptic meningitis may occur in patients with other auto-immune conditions such as systemic lupus erythematosus or mixed connective tissue disease. The symptoms of aseptic meningitis are: a very high temperature, being sick, a headache, a blotchy rash that does not fade when a glass is rolled over it (this may not develop), a stiff neck, a dislike of bright lights, drowsiness and fits.
Other reported side effects are:
Sensory disorders such as headache, ringing or buzzing in ears, dizziness, abnormal vision, hallucinations, tingling, pricking and burning of the skin (pins and needles) and vertigo (a sensation that objects are moving or spinning). Gastrointestinal problems such as mouth ulcers, sore mouth, nausea, vomiting, stomach upsets, diarrhoea, constipation, wind, heartburn, indigestion. Skin disorders such as swelling of tissues, itching of the skin, rash, redness. General disorders such as fever, drowsiness, tiredness, weakness, sleeplessness, shaking, nervousness, depression, confusion. Occasionally the blood does not clot well, which may result in easy bruising or bleeding. Rarely, a severe reduction in the number of white blood cells, which makes infections more likely.
Other rare side effects like shortness of breath
If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician
5. How to store Setolac®-P Tablets
Keep out of reach of children. Protect from light and moisture. Setolac®-P Tablets should be kept at room temperature (below 300C).
6. Contents of the pack and other information
What Setolac®-P Tablets contains
Each uncoated tablet contains
S (+) Etodolac 200 mg
Paracetamol IP 325 mg
Excipients q.s.
What Setolac®-P Tablets looks like and contents of the pack